KRF Clinical Practice Guidelines in Keloid Disorder (KRF Guidelines®) Chest Keloids Version 1.2019
Michael H. Tirgan, MD
SUMMARY
This guideline reviews the clinical presentation and treatment of anterior chest keloids. The sheer size of the anterior chest skin and the commonality of keloids in this area provides us with a unique opportunity to better understand the clinical characteristics and biology of keloid disorder.
Diagnosis
Diagnosis of anterior chest keloid lesions is based on clinical history as well as clinical appearance of the skin lesion(s). A biopsy is almost never indicated to establish the diagnosis of chest keloids. Grouping of anterior chest keloids
For purposes of this Guideline, chest keloid lesions are divided into six distinct groups:
- Early-stage lesions presenting as protruding papules, linear and nodular lesions (<2 cm – Stage IA).
- Locally advanced / multi-focal or organized patches presenting as a conglomeration of papules or linear lesions and the formation of keloid patches.
- Tumoral disease presenting as a bulky tumor mass(es).
- Tumoral – IB (2-5 cm – bulky tumor mass, Stage IB)
- Tumoral – semi-massive (5-10 cm – bulky tumor mass, Stage IC)
- Tumoral – massive (>10 cm – bulky tumor mass, Stage IIA and higher)
- Diffuse, widespread anterior chest keloid (DWACK) syndrome.
- Superficially Spreading Keloids presenting as large areas of skin involvement (> 10 cm in diameter – Stage IC and above).
- Post sternotomy keloids.
Treatment
Among all keloids, anterior chest keloids are the most difficult keloids to treat. The choice of treatment tools for these keloids is dependent on the size and the extent of the disease:
- Intra-lesional triamcinolone (ILT) shall be the first-line treatment for all early-stage, papular, and linear lesions (see KRF Guideline – ILT).
- Intra-lesional chemotherapy (ILC) should be considered for all early-stage, papular, and linear lesions that fail to respond to ILT (see KRF Guideline – ILC).
- Contact-cryotherapy with or without ILT/ILC is the preferred and primary method of destruction of all nodular, locally advanced, and tumoral keloid lesions (see KRF Guideline – Cryotherapy).
Rationale for the use of cryotherapy:
a. Cryotherapy is an effective method of treatment for protruding and bulky keloids.
b. As opposed to surgery, cryotherapy does not cause the worsening of keloids.
c. As opposed to surgery, radiation therapy is unnecessary after cryotherapy [1,3,4].
Treatments to avoid
Surgery shall not be used in the treatment of anterior chest keloids. The disease process in majority of patients is multifocal and progressive. Furthermore, surgical intervention is a known cause for the worsening of keloids [1, 2], which is also well documented in several cases presented in this Guideline.
Radiation therapy shall not be used in the treatment of chest keloids. This intervention may induce neoplastic transformation of irradiated tissues [3,4].
Lasers shall not be used in treatment of chest keloids. This intervention may result in the worsening of keloids [5].
OVERVIEW
This KRF Guideline was developed with the aim to provide:
- General discussion of chest keloids.
- Natural history of chest keloids.
- Classification system for chest keloids.
- Recommendations for treatment and follow up.
Introduction
With rare exceptions, keloid involvement of the anterior chest skin is dynamic and multifocal even at very early stages of the disease. Although there are many similarities between keloids of the chest, shoulders, and upper arms (CSUA), treatment for each set of keloids will be reviewed in separate Guidelines. Chest skin is the most common region of the body to develop keloids. In an analysis of geographical distribution of keloid lesions among 1088 patients, 484 patients (44.4%) had keloid involvement of the chest area in contrast to 298 patients (27.5%) with shoulder keloids and 177 patients (16.4%) with keloids in their upper arm area (see Table 1). As most patients have keloids in various parts of their skin, collectively 593 patients (54.5%) had at least one keloid lesion either on their chest, shoulder, or upper arm regions.
Table 1. Distribution of keloid lesions among a cohort of 1088 patients.

When broken down by disease site (Table 2), there seems to be minimal difference in the demographic characteristics of patients with any form of CSUA keloid. However, clear differences emerge when the analysis focuses on the disease site. This analysis showed that in only 50% of the patients who presented with CSUA keloids was the disease only limited to the CSUA skin; in the remaining patients, keloid lesions were present elsewhere on the skin.
An analysis of the patients who presented with chest keloids revealed that in only 31% of these patients was the disease limited to the chest at the time of presentation. In the other 69%, the disease was present elsewhere on their skin. A much stronger association was seen among patients who presented with shoulder keloids. The disease was limited to the shoulder area in only 11.1% of these patients; the remaining 88.9% had disease elsewhere on their skin. Most noticeably, in patients who present with upper armonly disease, the disease is limited to this site in 7.9% of the patients; the remaining 92.1% of patients have disease elsewhere on their skin.
Clinical presentation of keloid disorder in the CSUA varies with ethnic background. The tumoral CSUA keloids are almost exclusively seen among African Americans. The most common triggering factor for the formation of CSUA keloids is acne. Thus, the proper management of acne should be incorporated into the plan of care for all CSUA keloids patients.
As with other keloids, the clinical presentation, size, and shape of CSUA lesions vary from patient to patient. CSUA keloids in their early-stages are small and few in number. As time passes, the lesions grow in size and spread to involve larger areas of the skin. Furthermore, clinical observation of patients with anterior chest keloids shows that the disease’s clinical presentation is an interplay among several factors, some of which are listed below.
Table 2. Demographics of patients and morphology of CSUA keloids.
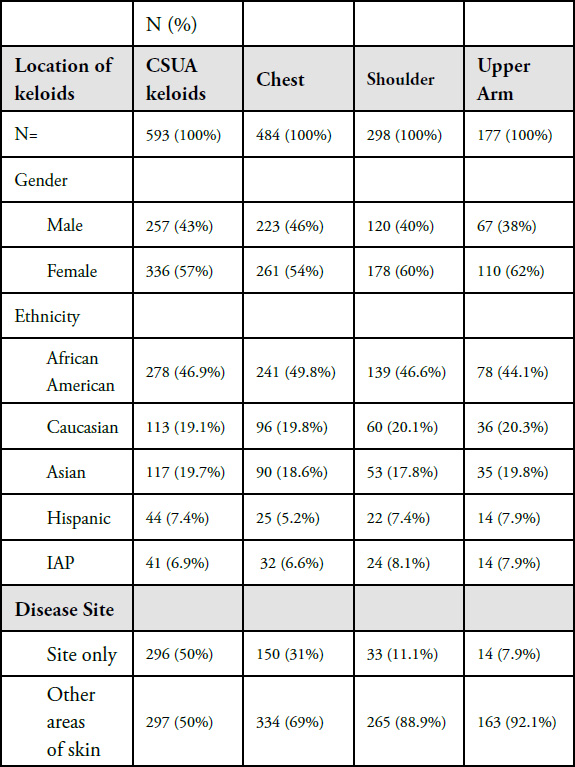
- Genetics of the underlying disorder is a fixed factor, which all patients are born with. Each patient is dealt a unique deck of genetic cards. Like most other genetic illnesses, there is a spectrum to the disorder’s genetic underpinning, spanning from a mild to moderate to severe form of the disease.
- Triggering factors play an essential role in the formation of keloid lesions. The intensity and the extent of the injury to the skin determines the severity and the extent of the clinical presentation of keloid disorder. Acne is perhaps the most common triggering factor in the formation of chest keloids. Other less common triggering factors are chicken pox, burn injury, or esthetic surgery.
- Passage of time is a factor for which all patients are equally exposed.
- Iatrogenic factors such as intralesional injections, laser treatments, and most importantly keloid removal surgery, can each result in a physicianinduced injury to either the keloid lesion being treated or the adjacent skin. These factors can result in formation of a much larger keloid at the treatment site. In most patients who suffer from the worst cases of keloid disorder, the prior keloid removal surgery unquestionably caused the formation of a much larger lesion and a worse outcome.
Careful observation and analysis of the clinical data identifies three distinct patterns in the development and progression of the disease in patients with anterior chest keloids.
- Group A. Patients who only develop one or a few small keloid lesions over an extended period of time (five to ten years) and their disease follows a very slow pace. Examples are seen in Case Studies 1-4 below.
- Group B: Patients in whom the disease has proceeds at a continuous pace and over time, the disease progresses, new lesions constantly form, and existing lesions grow in size.
- Group C. Patients who develop several lesions over a short period of time and by removal or resolution of the triggering factor (often acne), the disease process slows and no more lesions are formed (DWACK syndrome).
The clinical data of all 484 patients with chest keloids is now undergoing detailed analysis to determine the prevalence of different types of chest keloids, and the prevalence of each type of keloid among individuals of different ethnic backgrounds. Further analysis is also needed to determine the relationship between surgery and development of tumoral keloids. This analysis will be included in the next update to this Guideline.
Overall Treatment Strategy
A few basic principles must be considered in planning to treat all keloid patient, especially those with chest keloids:
- Involvement of the chest skin is a dynamic process. Over time, specially at the onset, the disease will progress in most patients and will result in the enlargement of existing lesions.
- The disease process is multifocal. Quite often patients will form new lesions, either near their original site of a chest keloid(s) or distant from it and in other parts of their skin.
The most important goals of treatment are:
a. To bring the triggering factor, i.e. acne, under control.
b. To intervene early and to bring the lesions under control with a combination of intra-lesional triamcinolone (ILT) / intra-lesional chemotherapy (ILC) and cryotherapy.
c. To avoid surgery in all patients.
EARLY-STAGE ANTERIOR CHEST KELOIDS
Anterior chest keloids at their earliest stages appear in three distinct manners:
- Protruding papule(s) that are often triggered by acne (figures 1, 3). If left untreated, these lesions can grow to become nodular keloids, or merge with adjacent keloid papules to form linear keloids (figures 7-11).
- Linear lesions that either develop as a result of a liner injury to the skin (figures 4, 5) or the merger of adjacent papules (figures 7-11). If left untreated, these lesions can grow to form thicker linear lesions or form keloid tumors (figures 35-47).
- Nodular lesions, which have either a clear and well demarcated circumferential border (Figure 6) or an active inflammatory margin (Figure 12, 64). This very elemental difference in the appearance of the keloid nodules is most probably due to biological differences in the two keloids. If left untreated, these lesions can grow to form thicker or bulky tumoral keloids.
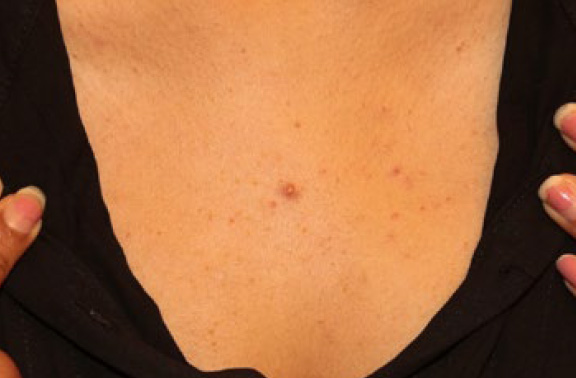
Figure 1. Early-stage papular chest keloid in a 22-year-old Asian female with a three-year history of keloid disorder.
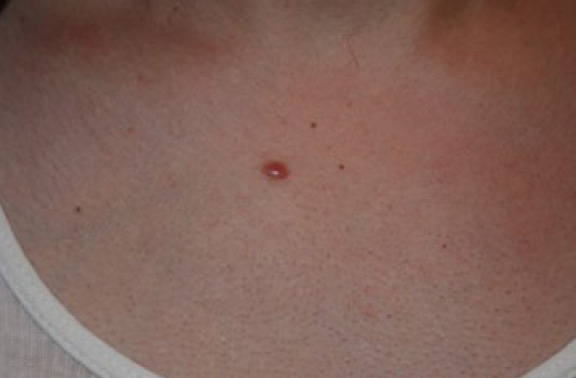
Figure 2. A 27-year-old Caucasian male with a single anterior keloid papule present for two years.
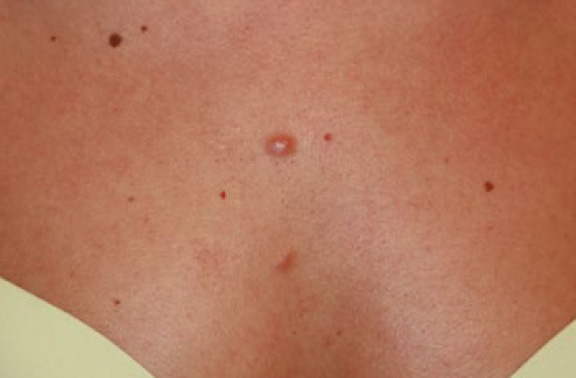
Figure 3. Early-stage keloid papules in a 28-year-old Caucasian female with an eight-year history of keloid disorder involving her chest and shoulders. Note the presence of two midline keloid papules.
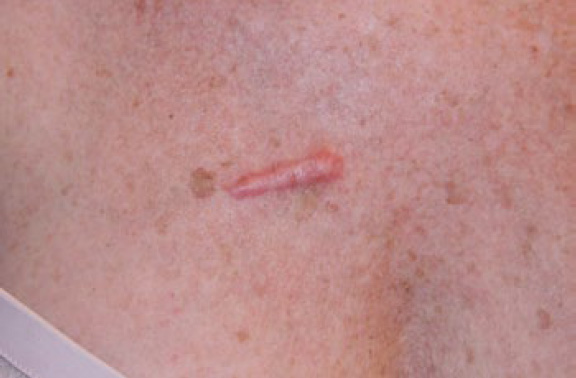
Figure 4. Linear chest keloid in a 53-year-old Caucasian female.
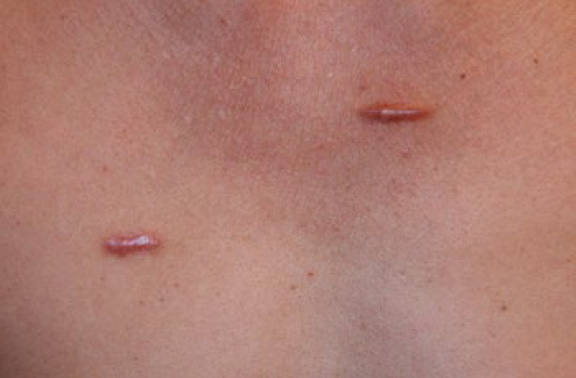
Figure 5. Two linear chest keloids in a 51-year-old Hispanic male.
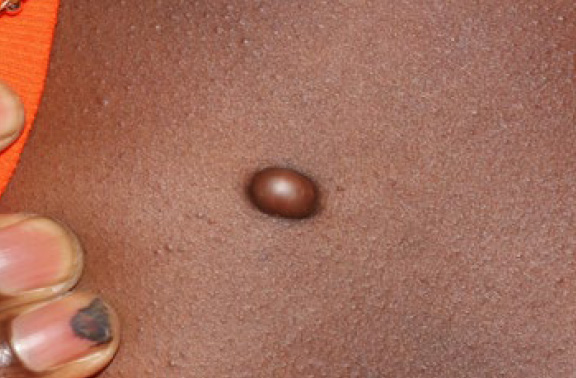
Figure 6. Anterior chest keloid nodule in a 15-year-old African-American male. Note the sharp and clear circumferential border of this keloid.
Case Study 1
A 25-year-old Asian female presented in June 2015 for treatment of multiple nodular facial keloids and several papular anterior chest keloids (Figure 7). It was interesting to note that these chest lesions, although papular, had all formed along an almost horizontal line.
During the course of treatment for her facial keloids, the chest keloids were monitored and serially photographed. In February 2016, after eight months of follow up, the chest keloids had grown in size and two medial keloid papules had grown towards each other (Figure 8).
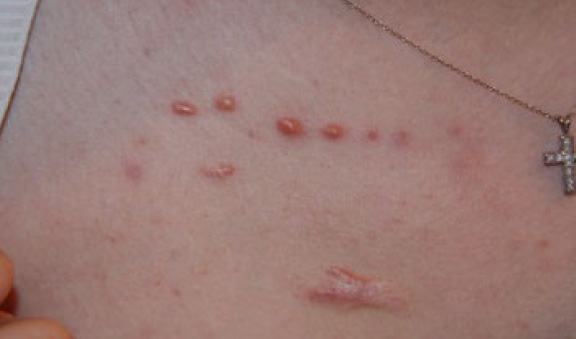
Figure 7. A 25-year-old Asian female who presented with multiple facial and several papular chest keloids (June 2015).
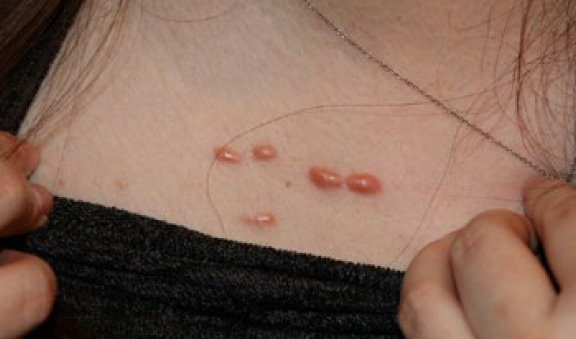
Figure 8. The same patient, February 2016. Note that within the span of eight months the two medial keloid papules have grown in size and are growing towards each other.
By May 2016, close to one year from initial presentation, the two medial keloid papules had merged, forming a linear keloid (Figure 9). However, in the same time frame, the two lateral lesions that were just inches away from the linear keloid followed a different path and did not merge. At this point, since the patient was receiving ILC for treatment of her facial keloids, treatment with ILC was also started for all chest lesions. Over the next several months, the two lateral papules showed a dramatic response to treatment, however the linear keloid exhibited resistance to this treatment (figures 10-11).
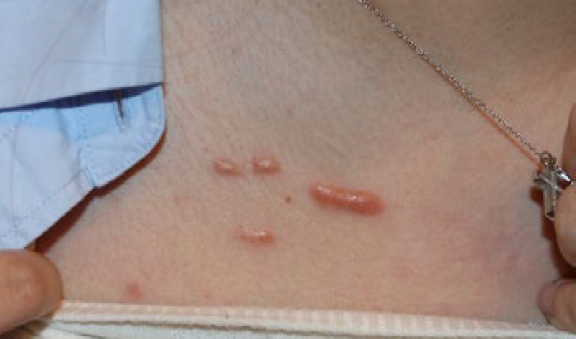
Figure 9. The same patient, May 2016. Note that in span of one year since the initial presentation, the two medial papules have grown in size and merged to form a linear keloid. The lateral lesions have not merged.
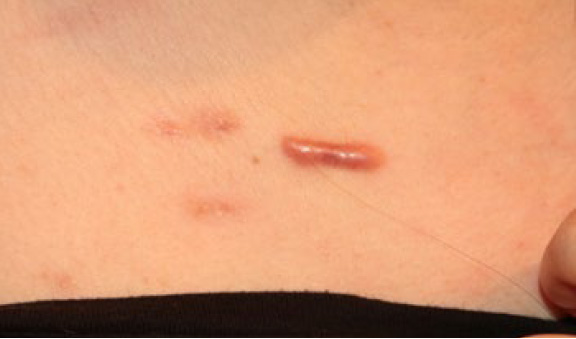
Figure 10. The same patient in August 2018. Note that in a span of five months since initiation of treatment with ILC, the linear keloid remains active and exhibiting resistance to treatment, yet the two lateral papular lesions have responded to treatment and have almost completely regressed.
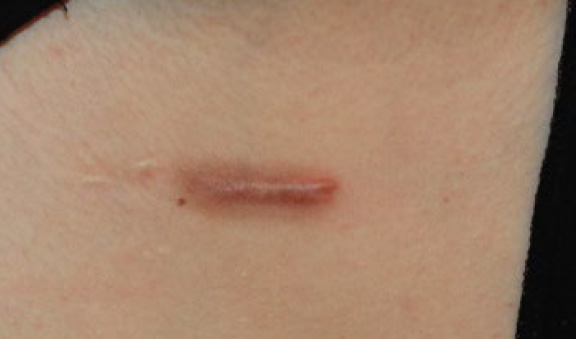
Figure 11. The same patient, December 2018. Note that in span of two and a half years, after several ILC treatments, the linear keloid still remains active and somewhat resistant to treatment, yet the two lateral papular lesions have completely regressed. The hyperpigmentation around the linear keloid is an expected reaction to the repeated ILC injections.
This case illustrates the process of formation of the linear keloids. At least in some patients, the merger of two adjacent papular keloids may indeed be the process for formation of linear keloid.
Furthermore, the clinical observation of this patient’s keloid papules – over the span of two and a half years – provides evidence that similarly appearing keloid lesions, even in the same patient and even if they are in the same general vicinity, can biologically behave differently and progress along completely different paths.
As demonstrated above, the two pairs of keloid papules also exhibited different levels of responsiveness to ILC. The lateral papular lesions had a dramatic response to ILC, but the linear lesion proved to be rather resistant to the same treatment.
Highly Variable Clinical Presentation of Early-Stage Disease
Although the initial clinical presentation of keloid disorder in most patients is similar, i.e. development of small keloid papules, the disease entity has a diverse biology. Over time, the disease can result in a highly variable clinical presentation, including numerous distribution patterns, variable growth rates, and variable responses to similar treatments. As case study 1 shows, heterogeneity in response to the same treatment is also observed among similarly appearing lesions in the same patient.
The variability in the biological behavior of the disorder must be taken into consideration when treating keloid patients.
Treatment
There are two distinct goals for treatment of patients with early-stage anterior chest keloids:
- To induce remission.
- To prevent progression or worsening of the lesions.
It is of the utmost importance to manage early-stage keloid lesions in a manner that prevents them from transforming into large and tumoral keloids. Both the treating physician and the patient must also be cognizant that the disorder is multifocal with a dynamic pathophysiology. While the triggering factors are still active, with the passage of time the existing lesions will continue to grow in size. As this Guideline documents, most patients are destined to develop new lesions, either in the same vicinity, or elsewhere on the chest or distant parts of their skin. It is naïve to think that the keloid process is static or limited only to one segment of the skin such that it can be surgically removed. All keloid patients must be treated in accordance with a long-term treatment and follow-up plan. Patients also need to be adequately educated about the nature of their illness and its biology.
ILT injection shall be the first line of treatment for all earlystage papular/linear chest keloid lesions. For lesions that respond to this treatment, ILT shall be continued on a regular basis to achieve maximum response. Of equal importance, however, is to identify and to treat the triggering factors. In the case of acne, anti-acne treatment shall be optimized. ILC should be considered early on for all lesions that fail to respond to ILT.
Contact cryotherapy shall be incorporated into the management of all nodular or bulky chest keloids. Residual keloid tissue after cryotherapy shall be treated with ILT and/or ILC.
Case Study 2
A 20-year-old Caucasian female presented with a solitary, previously untreated two-centimeter (cm) central chest keloid (Figure 12). She had no other keloid lesions on her skin. This lesion was treated with ILT and entered a complete and durable remission in two months after receiving only three injections (Figure 13).
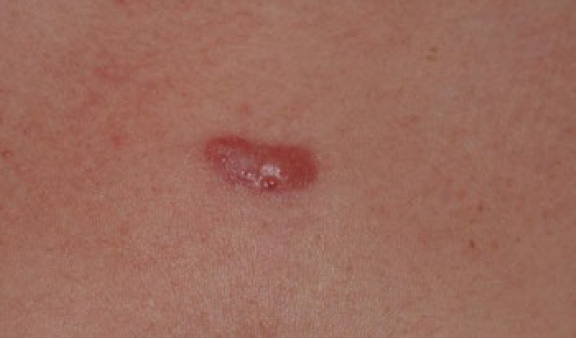
Figure 12. Previously untreated solitary anterior chest keloid in a 20-year-old Caucasian female.
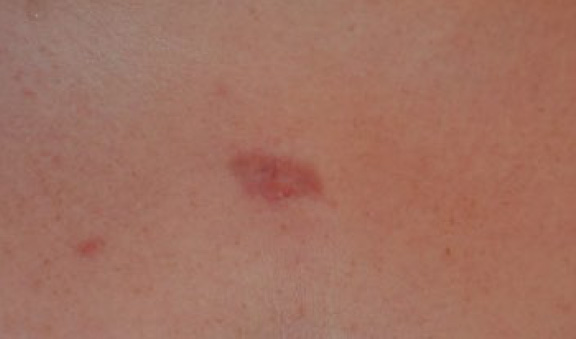
Figure 13. The same patient after receiving three doses of ILT. Note the total flattening of the keloid two months after initiation of treatment.
Case Study 3
A 26-year-old Caucasian male presented with a five-year history of a solitary central chest keloid that failed to respond to ILT injections (Figure 14). The lesion was treated with a combination of ILT and cryotherapy. A durable complete remission was achieved one year later, after five cycles of combined treatment (Figure 15).
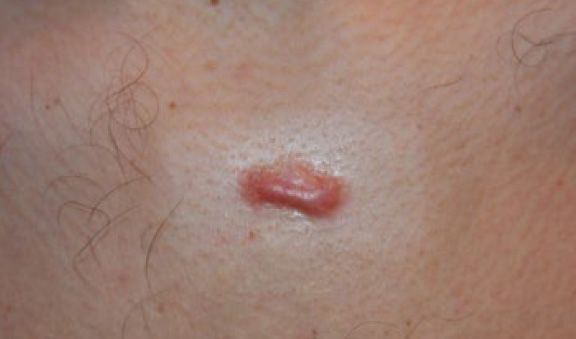
Figure 14. Solitary anterior chest keloid in a 26-year-old male. Note the inflammatory margin of this lesion.
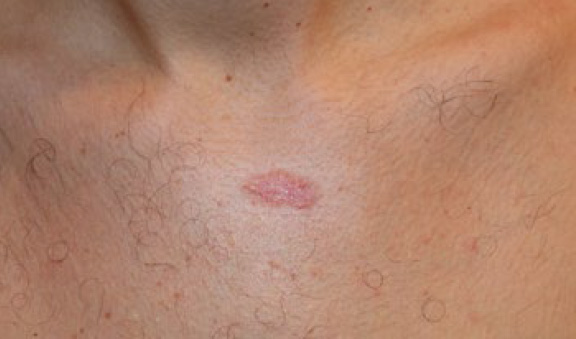
Figure 15. The same patient after receiving five cycles of ILT and contact cryotherapy. Note the total flattening of the keloid one year after initiation of the treatment.
Case Study 4
A 20-year-old Caucasian female presented in May 2012 to the author with three papular central chest keloids (Figure 16). Her first chest keloid papule had developed two years earlier and had failed to respond to ILT. The lesion was then surgically removed. There was a recurrence shortly after the surgery and it failed to respond to repeated treatments with ILT.
During the initial visit in May 2012, the patient was educated about the natural history of the disorder. She returned for treatment in September 2012 (Figure 17). Within four months, there was minimal progression with a slight increase in the erythema and slight growth in the two adjacent upper chest lesions. During this visit, all lesions were treated with contact cryotherapy (Figure 18).
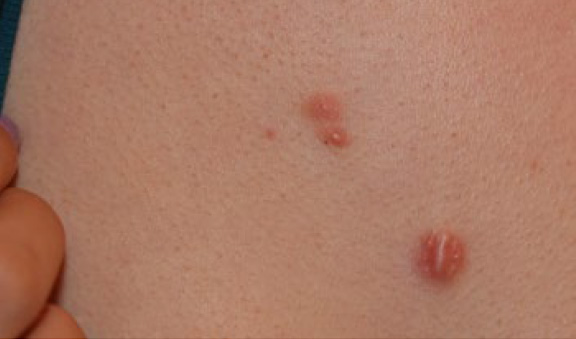
Figure 16. Initial presentation of a 20-year-old Caucasian female with a two-year history of struggling with chest keloids (May 2012). Note the inflammatory reaction around the margins of all three lesions.
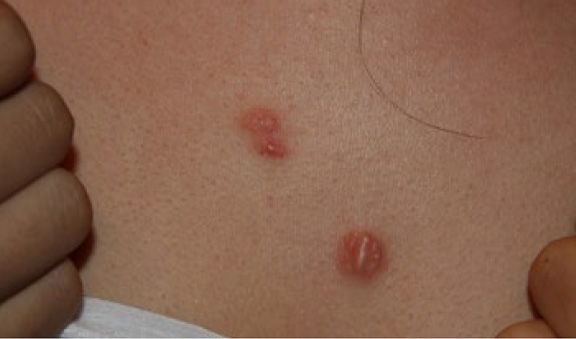
Figure 17. The same patient presented four months later (September 2012). Note the increased erythema and minor progression of the two adjacent lesions.
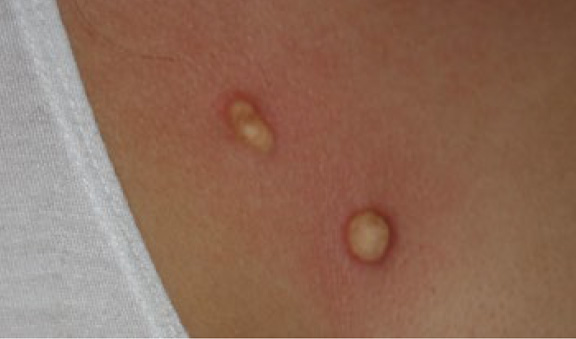
Figure 18. The same patient immediately after application of contact cryotherapy (September 2012). Frozen tissue appears white in color.
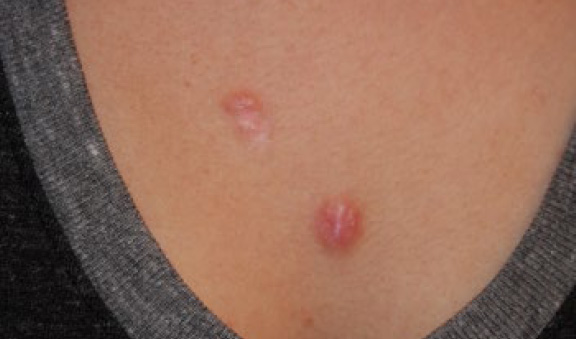
Figure 19. The same patient two months after treatment with contact cryotherapy (November 2012).
The patient returned two months later. There was some improvement in the appearance of all lesions (Figure 19). At this point, ILT was added to contact cryotherapy and the treatment was repeated on a monthly basis. By January 2013 (Figure 20), and after total of three treatment cycles, the two merging lesions had somewhat responded to treatment and had nearly disappeared. Yet the larger lesion, although it had responded to treatment, was still present.
By June 2013 (Figure 21), the merging lesions had responded to treatment with only one lesion persisting. The patient was offered ILC, yet declined treatment and chose to pursue laser treatments elsewhere.
After a long gap in follow up, the patient returned in December 2017 (Figure 22) for repeat consultation and to consider ILC. At this time, the two merging lesions were no longer present yet the larger lesion had grown in size. Since her last visit in June 2013, the patient had undergone multiple ILT and several laser treatments. At this time, the lesion was injected with 2 micrograms of intra-lesional vincristine (IL-VCR), with a significant response. Figure 23 depicts the appearance of this lesion after two IL-VCR treatments (December 2018). The patient was then 26 years of age and had struggled with keloid disorder since she was 18 years of age.

Figure 20. The same patient after three cycles of treatment (January 2013). Note near total ablation of the two merging lesions and reduction in the mass of the larger lesion.
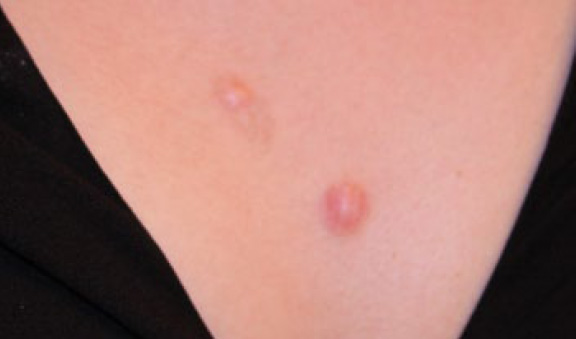
Figure 21. The same patient after five cycles of treatment (June 2013). Note near total ablation of the two merging lesions but persistence of the larger lesion.
This patient exemplifies the challenges of treating earlystage chest keloids and the variable responsiveness of adjacent lesions to the same treatment. Despite the fact that two smaller lesions responded to treatment with ILT and cryotherapy, the larger lesion did not. This lesion was originally treated with several cycles of ILT with no appreciable response and then underwent surgery but regrew soon after resection. The recurrent lesion had a partial response to ILT and cryotherapy but failed to respond to a subsequent laser treatment. This lesion eventually responded to IL-VCR.
Another interesting observation in this patient was that during the span of six years, this patient did not develop any new lesions on her anterior chest.
Furthermore, as noted in the four cases presented thus far, the disease characteristics and the response to treatment in each patient has been very different. These differences are possibly due to genetic and biological differences that remain unknown to this date.
Progression of the Early-Stage Chest Keloids
In most patients, over time each early-stage keloid lesion will grow in size to involve wider areas of the surrounding skin. Most (but not all) patients will also develop new lesions, often in the vicinity of the original lesion(s), or further away and in distant skin regions. Progressive disease in one area is often associated with progression of the disease elsewhere on the skin. Figures 24-29 depict several examples of gradually advancing anterior chest keloid lesions.
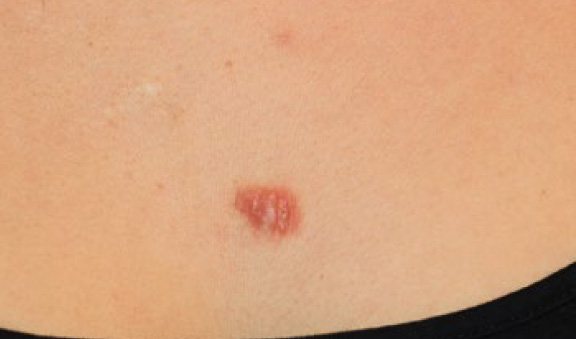
Figure 22. The same patient, five years after initial presentation (December 2017). Note the complete regression of the two merging lesions that were treated with ILT and cryotherapy in 2012-2013. The larger lesion is still present and slightly larger compared with the initial presentation in 2012.
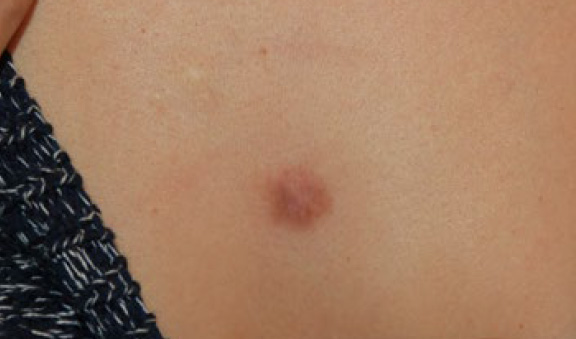
Figure 23. The same patient after two doses of IL-VCR (December 2018). Note the significant reduction in the bulk and mass of the large lesion.
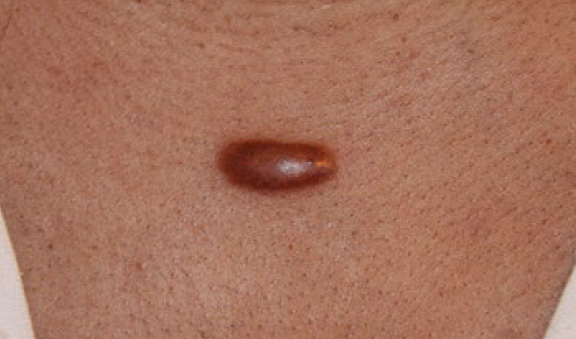
Figure 24. Untreated thick linear anterior chest keloid in a 39-year-old African-American female.
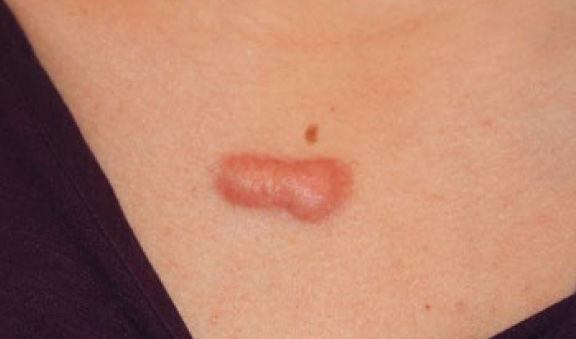
Figure 25. Thick linear anterior chest keloid in a young Caucasian female. Note the inflammatory border around the keloid.
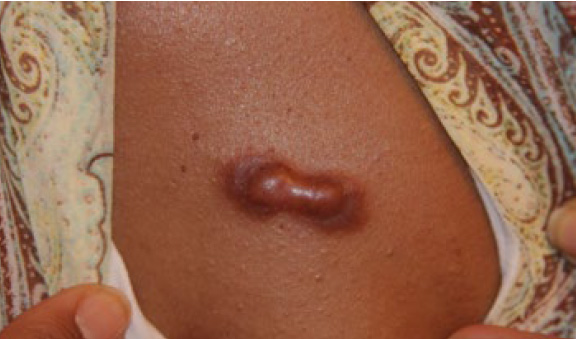
Figure 26. Thick linear/nodular anterior chest keloid in a 36-yearold African-American female who has been struggling with keloid disorder since she was 13 years old.
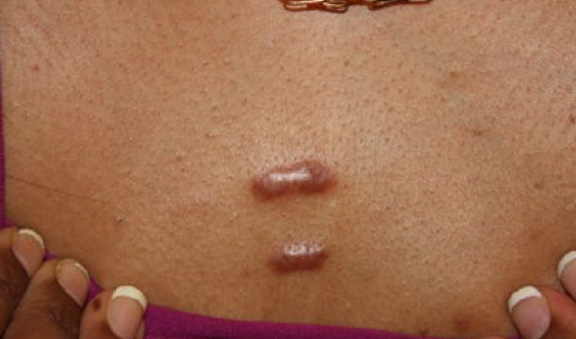
Figure 27. Thick linear keloids of the anterior chest in a 38-year-old Indian female who has struggled with keloid disorder since she was 27.
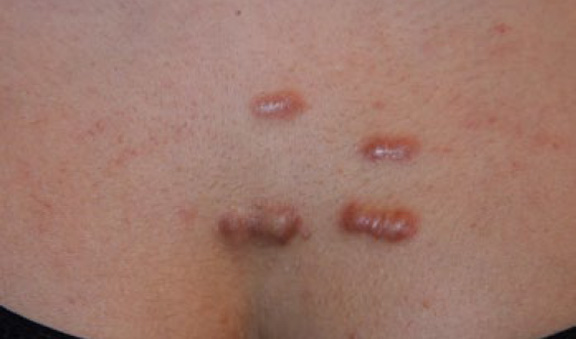
Figure 28. Multiple thick linear anterior chest keloids in a 24-yearold Indian female who has struggled with keloids for three years.
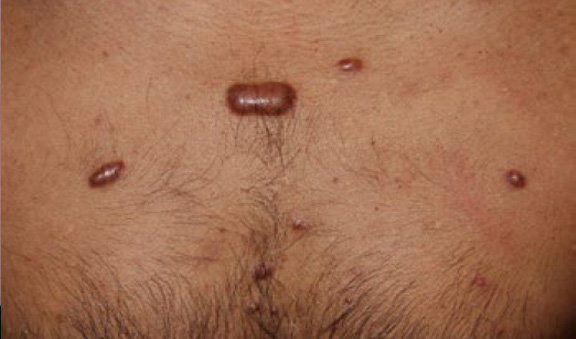
Figure 29. Multiple thick linear and papular keloids of chest in a young Indian male.
Treatment Strategy
As evidenced by the disease’s multifocality and since the triggering factor for formation of keloid lesions is injury to the skin, it is logical to conclude that the treatment approach to this illness must be non-surgical, using methods that do not trigger a repeat keloidal wound healing reaction. As is evidenced later in this Guideline, approaching keloid disorder with surgery, and chest keloids in particular, can result in a much worse outcome. Non-surgical treatments using ILT, ILC, and cryotherapy, although not ideal, can indeed produce significant results, especially when introduced early on in the course of the illness, in particular in patients with one or a few keloid lesions.
Case Study 5
A 29-year-old African-American male presented on April 5, 2014, with a solitary three centimeters wide anterior chest tumoral keloid (Figure 30). This keloid started as a solitary papule when the patient was 16 years of age. Although the lesion had gradually grown in size, it remained asymptomatic until a few months prior to this presentation when the patient noticed pruritis. Due to this keloid’s slow rate of the growth, and fear of making it worse, the patient had chosen to postpone treatment until the keloid became symptomatic.
Treatment was started using ILT given once every three to four weeks to improve the symptoms and to maximally flatten the lesion. Although ILT injections resulted in improvement in pruritis, the keloid lesions showed no evidence of physical response after three scheduled ILT injections. At this point, cryotherapy was introduced as a second-line treatment to reduce the bulk of the lesion (May 2014). Figure 31 depicts the appearance of the keloid in October 2014 after recovery from two cycles of contact cryotherapy.
Although cryotherapy can induce partial debulking of tumoral anterior chest keloids, often will not result in total or near total remission. This is in total contrast with how successfully cryotherapy works for ear keloids (see KRF Guideline – Ear Keloids). In October 2014, ILC was introduced for the remnant of this keloid. The patient received monthly injections of ILVCR and docetaxel. Near complete remission and flattening of the keloid was achieved by March 2015 (Figure 32).
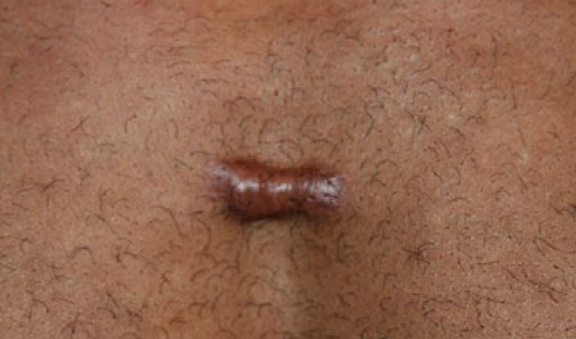
Figure 30. A 29-year-old African-American male who presented with a three centimeter, previously untreated anterior chest wall keloid (April 5, 2014).
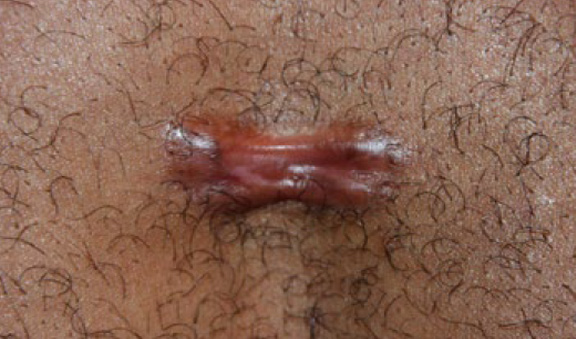
Figure 31. The same patient after receiving two cycles of contact cryotherapy (October 2014).
With significant reduction in the mass of this keloid, the patient entered a maintenance phase of treatment: he returned once every six to nine months for repeat ILC treatment using IL-VCR at doses of 2 micrograms per session. Figure 33 depicts the appearance of this keloid at the last follow up visit in July 2018.
This case study shows that central chest keloids can be successfully treated with a combination of ILT, cryotherapy, and ILC. Once the disease is under reasonable control, patients must be followed on a regular basis. The recurrent disease must be treated with the last successful method of treatment.
LOCALLY ADVANCED / MULTI-FOCAL OR ORGANIZED KELOID PATCHES
In most, but not all patients, over time the disease process will gradually progress, resulting in the formation of larger keloid lesions, which tend to merge and form patches of the disease. Figures 34-37 depict four patients with this type of anterior chest keloids.
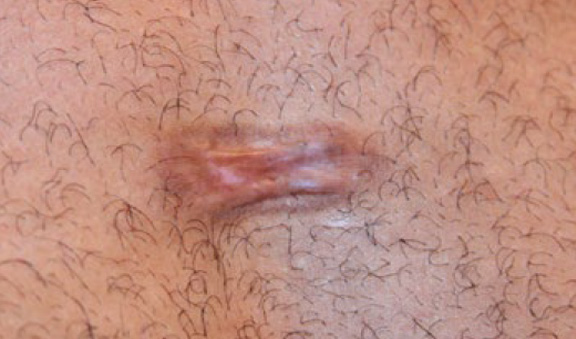
Figure 32. The same patient after three IL-CVR injections (March 2015).
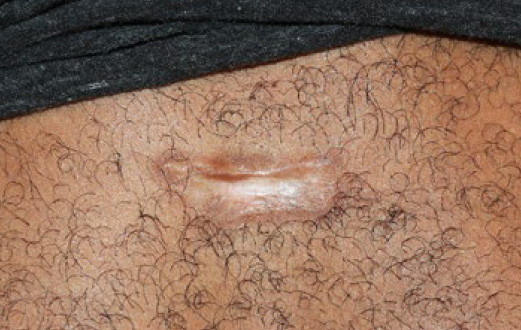
Figure 33. The same patient four years after initial presentation (July 2018). Patient returned for treatment of the linear recurrence on the upper edge of the keloid. The linear lesion was injected with 2 micrograms of vincristine.
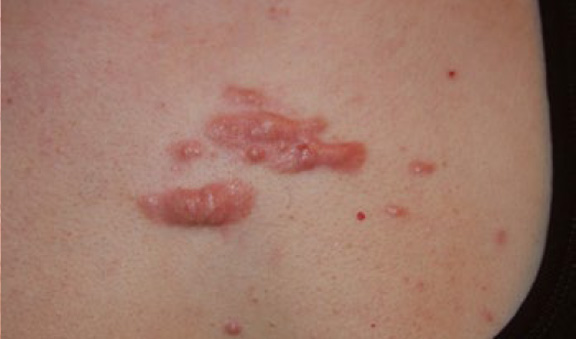
Figure 34. A 27-year-old Caucasian female with a ten-year history of widespread chest keloids. Note the multifocality of the keloids, merger and formation of keloidal patches, and inflammatory reaction around the larger lesions.
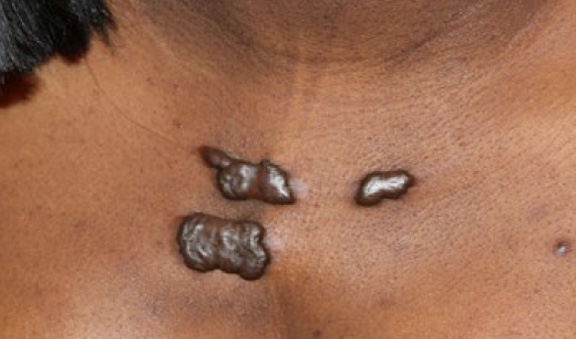
Figure 35. Multiple keloidal patches in a 28-year-old African-American female with a ten-year history of keloid disorder.
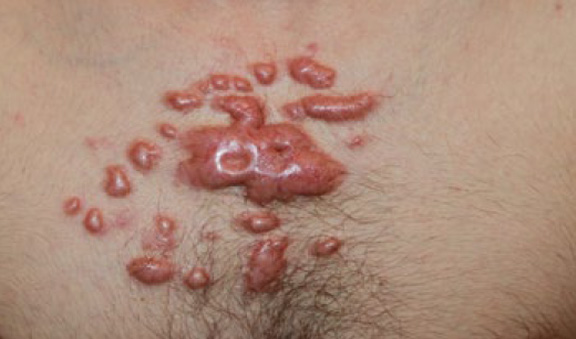
Figure 36. A 16-year old Caucasian male with a four-year history of widespread chest keloids triggered by acne. Note the merger and formation of the keloidal patch and the inflammatory reaction around most keloid lesions.
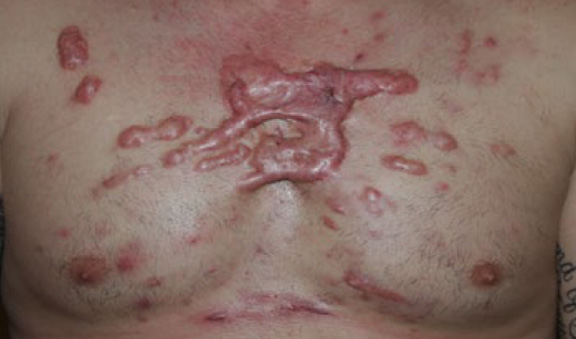
Figure 37. Widespread anterior chest keloids in a 29-year-old Caucasian male with a twelve-year history of keloid disorder.
TUMORAL KELOIDS
Unlike patients with multi-nodular keloids, a small subset of patients present with only one or a very few keloid lesions in the anterior chest area. Most of these patients present with a slow growing solitary anterior chest keloid (case studies 2, 3, and 5) without keloid lesions elsewhere on their skin. An untreated or surgically removed early-stage solitary chest keloid can potentially grow in size and expand three-dimensionally to form a large tumoral keloid. Large tumoral chest keloids, although they can be seen in Asians and Caucasians, are more often encountered in African Americans, especially in patients who have undergone keloid removal surgery.
The data on 484 patients with chest keloids will be analyzed to determine the prevalence of tumoral chest keloids among individuals with different ethnic backgrounds as well as the relationship between surgery and the development of tumoral keloids. This data will be included in the next update to this Guideline.
For purposes of this Guideline, tumoral chest keloid lesions are divided into three types:
a. Tumoral – IB (2-5 cm – bulky tumor mass, stage IB)
b. Tumoral – semi-massive (5-10 cm – bulky tumor mass, stage IC)
c. Tumoral – massive (>10 cm – Bulky tumor mass, stage IIA and above)
Figures 39-45 depict seven different patients with tumoral anterior chest keloids.
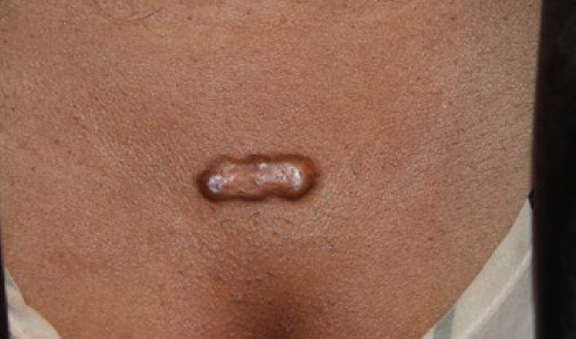
Figure 38. Solitary tumoral anterior chest keloid in a young African-American female.
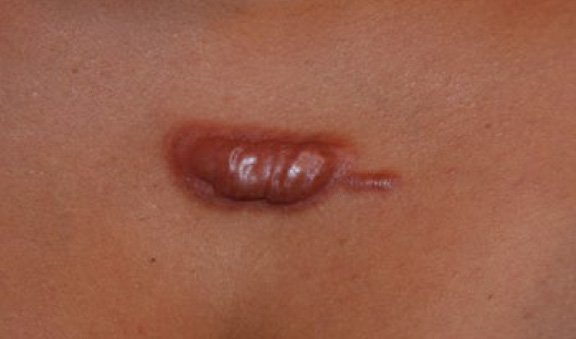
Figure 39. Thick keloidal tumor of anterior chest in a 34-yearold Hispanic female with an eleven-year history of keloid disorder involving her chest and shoulders. Note the formation of an earlystage linear keloid lesion in the left lateral margin of the main keloid.
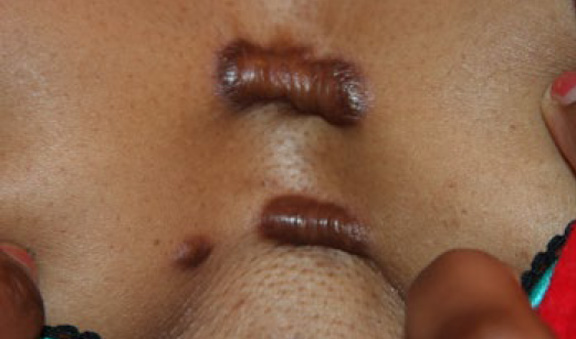
Figure 40. A 37-year-old African-American female with a fouryear history of anterior chest keloids. She developed three distinct keloids in close proximity to each other.
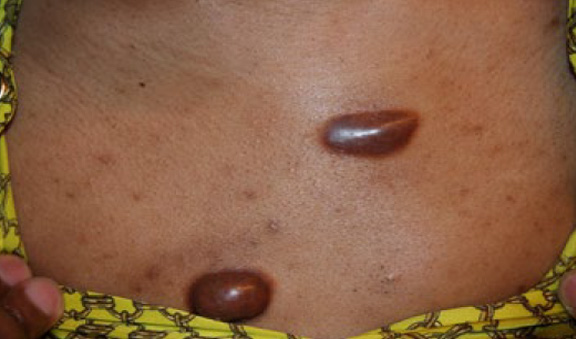
Figure 41. A 28-year-old African-American female with a tenyear history of struggling with ear, chest, and pubic keloids. Note the two distinct anterior chest keloids in close proximity to each other.
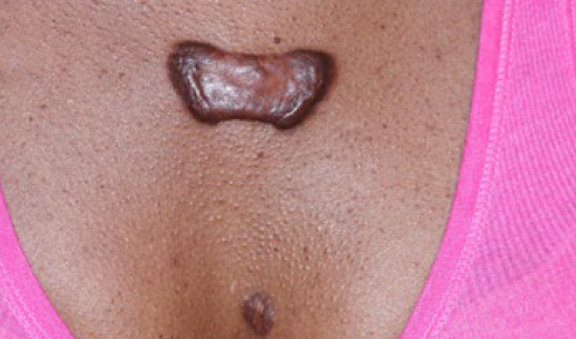
Figure 42. A butterfly-shaped tumoral central chest keloid lesion in a 30-year-old African-American female with an eight-year history of struggling with keloid disorder.
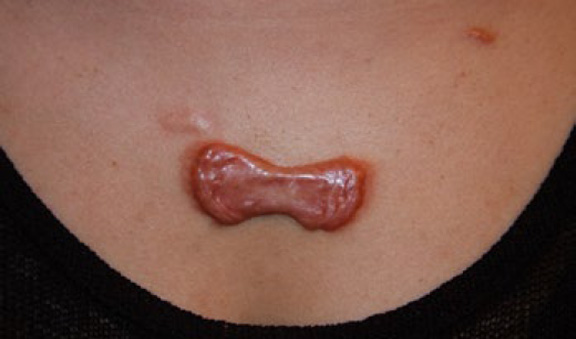
Figure 43. A painful, butterfly-shaped tumoral central chest keloid in a 30-year-old Asian female with an eight-year history of struggling with the keloid shown here. Note the multifocality of the keloid lesions and the inflammatory skin reaction at the lateral margins of the large keloid. Central chest keloids of this magnitude are almost always pruritic, tender, and painful to the gentlest touch.
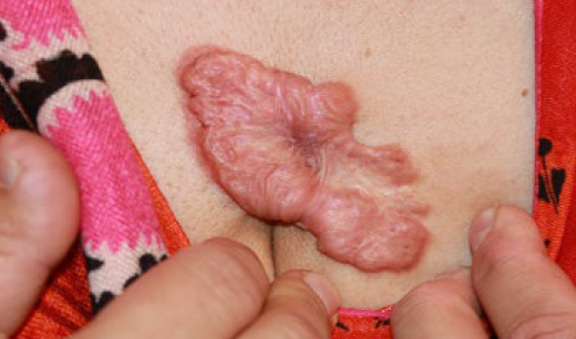
Figure 44. An irregularly shaped anterior chest tumoral keloid in a 42-year-old Middle Eastern female. This keloid first appeared as a small papule approximately twelve years earlier.
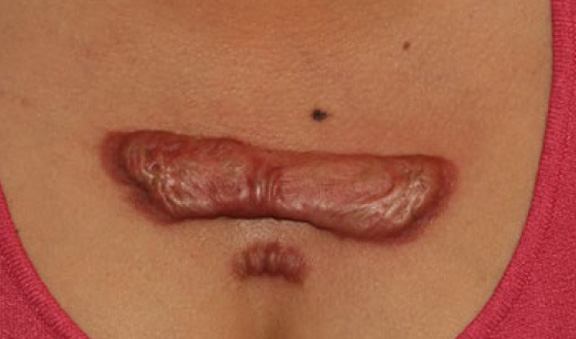
Figure 45. Large tumoral anterior chest keloid along with a smaller keloid in a 30-year-old female with an eleven-year history of struggling with chest keloid. Note the presence of intense inflammatory lateral margins.
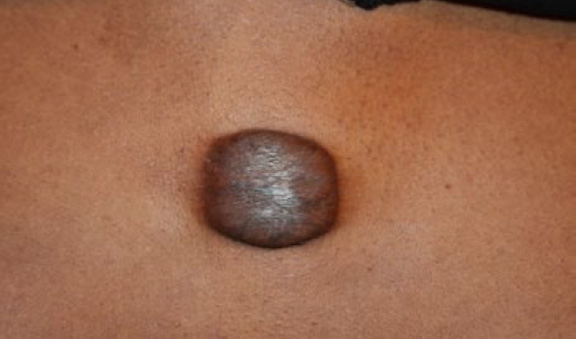
Figure 46. Large tumoral chest keloids at presentation (November 12, 2013).
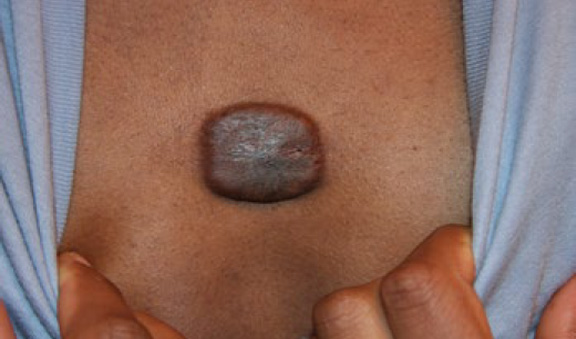
Figure 47. The same patient, two weeks after treatment with IL-VCR. Note reduction in the keloid’s mass and thickness (November 26, 2013).
Case Study 6
An 18-year-old African-American male presented in November 2013 with a 6×7 cm, almost round, tumoral central chest keloid (Figure 46). This lesion had first started when the patient was only six years old and had gradually progressed over time. Prior treatments were limited to a few ILT injections with no appreciable response. After a lengthy discussion concerning the various treatment options, the decision was made to treat this keloid with ILC. The keloid was injected with 10 micrograms of vincristine (see KRF Guidelines – ILC). Within two weeks, the keloid exhibited signs of responding to this treatment with a visible reduction in its mass and thickness (Figure 47). Treatment with ILVCR was continued every two to three weeks. Figures 48- 50 depict the results of the treatment.
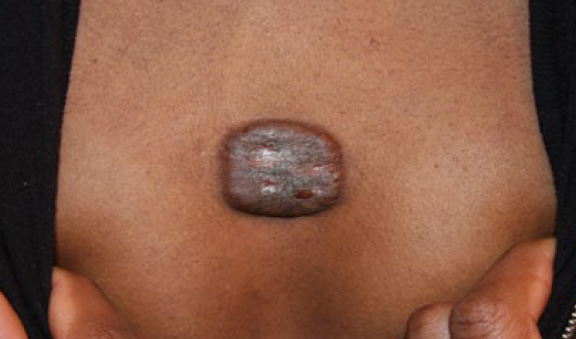
Figure 48. The same patient, four weeks after initial presentation, having received two courses of treatment with IL-VCR. Note further redction in the keloid’s mass (December 12, 2013).
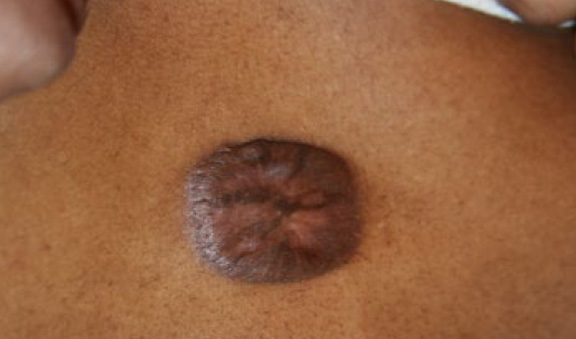
Figure 49. The same patient, two months after initial presentation, having received three treatments with IL-VCR. Note the further reduction in the keloid’s mass (January 7, 2014).
After the fourth treatment of IL-VCR, the patient was lost for follow up, but returned two years later in January 2016 with some regrowth of his keloid lesion (Figure 50). During this time, the patient had decided to undergo sex change treatments and surgery. After much discussion, the patient elected to be treated with cryotherapy in order to achieve a faster reduction in the keloid’s mass. The keloid was treated with contact cryotherapy during this visit (Figure 51), which resulted in significant debulking (Figure 52). Subsequently the patient received two more doses of ILVCR in March and May 2016.
By October 2016 (Figure 53) the keloid was much thinner. By March 2018 (Figure 54) , after several rounds of IL-VCR and Cryotherapy, the keloid was significantly less noticeable.
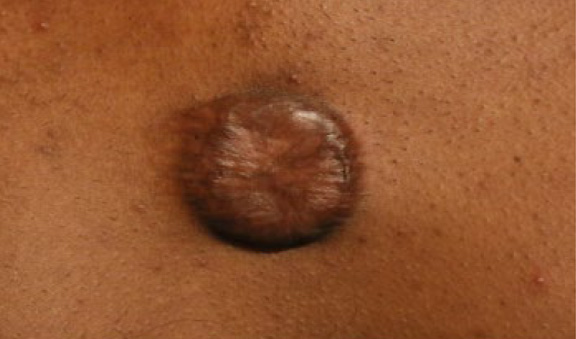
Figure 50. The same patient, two years after last treatment with IL-VCR. There was some regrowth of the keloid, yet the mass of keloid had remained smaller compared with the patient’s initial presentation (January 2016).
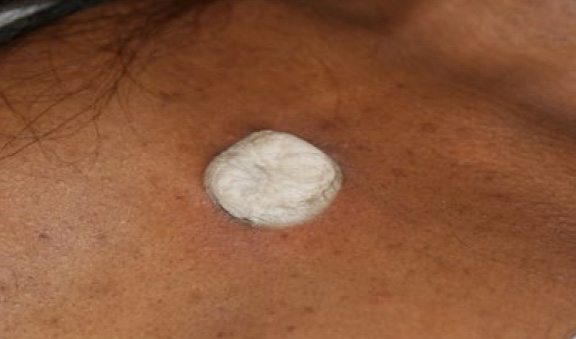
Figure 51. The same patient, immediately after application of contact cryotherapy (January 2016).
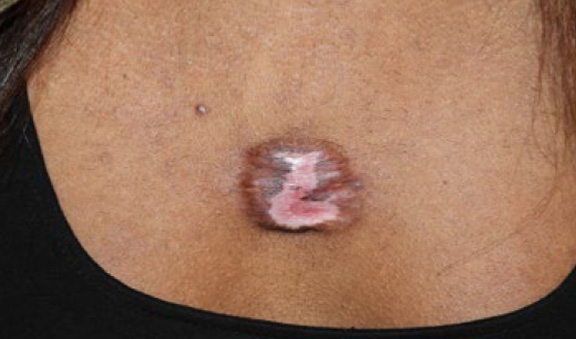
Figure 52. The same patient, four months after application of contact cryotherapy (May 2016). Note the reduction in the keloid’s mass.
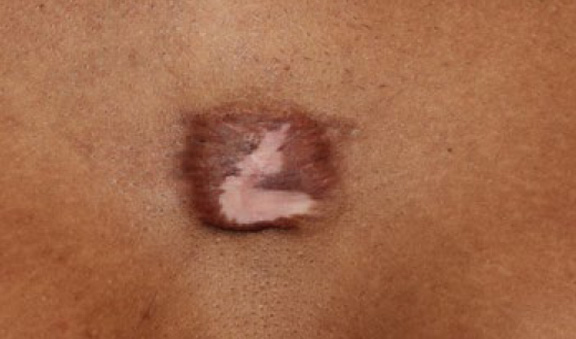
Figure 53. The same patient, ten months after application of one cycle of contact cryotherapy and two treatments with IL-VCR (October 6, 2016). Note the further reduction in the keloid’s mass.
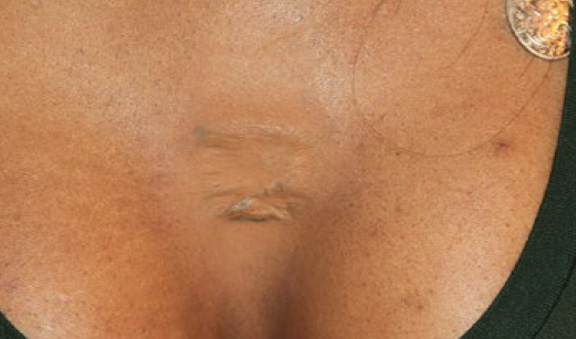
Figure 54. The same patient, after application of several cycles of contact cryotherapy and IL-VCR (March 1, 2018). Note the near flattening of the keloid. The cryotherapy’s induced loss of pigment is camouflaged with makeup. Although the results achieved are not ideal, they are significantly better than exposing this young patient to the risks associated with surgery and adjuvant radiation therapy.
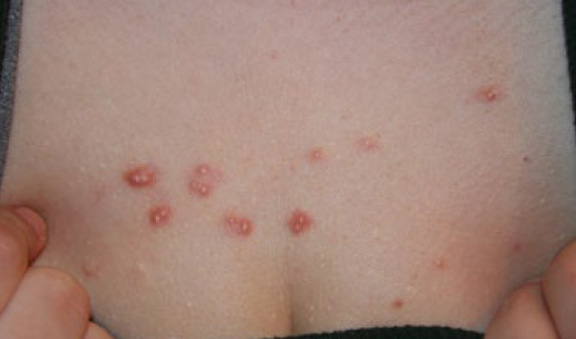
Figure 55. An 18-year-old Caucasian female with a two-year history of DWACK syndrome presenting with multiple papular keloids of the anterior chest and both shoulders (May 2014).
DIFFUSE, WIDESPREAD ANTERIOR CHEST KELOID (DWACK) SYNDROME
The heterogeneity in clinical presentation of keloid disorder is most noticeable in keloids of the anterior chest area as this region of the skin not only covers a large area, but also is the most common location for the development of keloid lesions.
Diffuse, widespread anterior chest keloid (DWACK) syndrome is a distinct clinical entity that presents with the formation of numerous papular or nodular keloid lesions over a short period of time. This syndrome is most commonly seen among young Caucasian patients and is almost always triggered by acne or treatment of acne with isotretinoin [6,7].
Heterogeneity in the intensity and duration of acne, treatment of acne, length of treatment with isotretinoin, and the underlying genetic propensity to develop keloids results in a highly variable clinical presentation of DWACK syndrome. The clinical presentation can range from patients who only form small papular lesions (Figure 55) to those with numerous nodular or tumoral keloids (figures 56, 57) in the anterior chest as well as shoulders and upper arms. Many DWACK syndrome patients develop all their keloids over a short period of time, after which time no new lesions form.
Treatment of these patients, especially those with numerous keloid lesions, is quite challenging. Small, papular lesions do tend to respond to ILT or ILC. Larger lesions, especially those that are bulky can be successfully debulked using cryotherapy. Pain control prior to application of cryotherapy to several large lesions will make the procedure tolerable and also ensures compliance with the treatment plan.
Careful treatment planning is paramount to proper management of these patients. Frequent ILT injections into numerous keloid lesions can expose these young patients to undue systemic toxicity from triamcinolone.
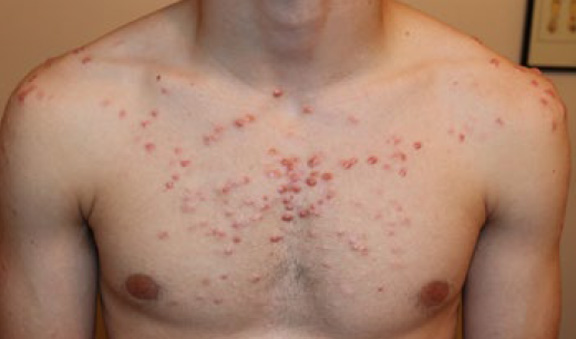
Figure 56. An18-year-old Caucasian male with a two-year history of DWACK syndrome, presenting with widespread anterior chest and shoulder keloids, triggered by acne that was treated with isotretinoin.
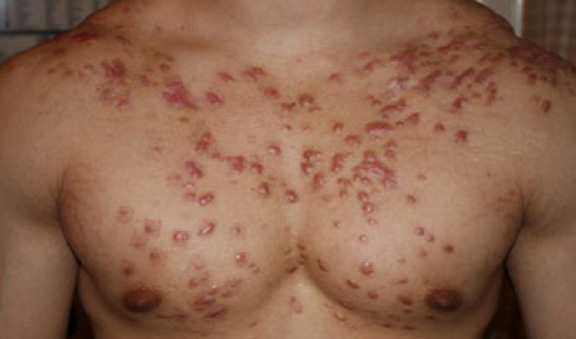
Figure 57. A 22-year-old Caucasian male with only a one-year history of DWACK syndrome, triggered by acne and presenting with widespread chest and shoulder keloids. Note that the neck area skin is spared from the keloid process.
SUPERFICIALLY SPREADING KELOIDS
Due to the vast heterogeneity in clinical presentation of the keloid disorder in the anterior chest area, there is a subset of patients whose disease will progress in a continuous and relentless manner, resulting in formation of large keloidal patches in the anterior chest skin. Although this process may be autonomous and seen in patients who have never undergone surgery (figures 58-60), there are many such patients whose disease takes on a much more aggressive course following keloid removal surgery.
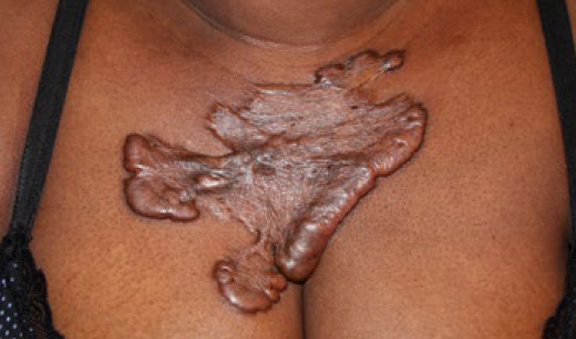
Figure 58. Superficially spreading anterior chest keloids in a 46-year-old African-American female with a six-year history of keloid disorder with a natural progression of the disease.
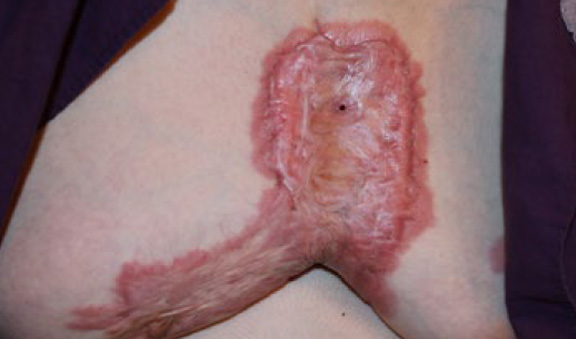
Figure 59. Locally advanced anterior chest keloid in a 55-year-old Caucasian female who has been struggleing with progressive anterior chest keloid since she was eleven years old. Note the presence of a fistula in the upper part of this keloid. Frequent deep infections are common complcations seen in patients with such thick aterior chest keloids.
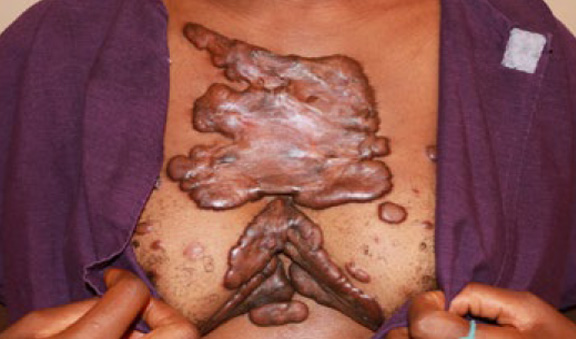
Figure 60. A 29-year-old female with extensive keloid involvement of anterior chest area. This patients first keloids started in her chest at the age of 14. Note the presence of numerous keloid lesions that are in various stages of development, including newly developed papules. Progression of the disease in this patient has been autonomous. She denied having had any prior surgeries on her chest.

Figure 61. A 42-year-old African-American female presented with a thick linear incisional keloid six months after sternotomy.
POST STERNOTOMY KELOIDS
Post sternotomy keloids are challenging complications of thoracic / cardiac surgery. It is obvious that the injury from surgery triggers this keloidal wound healing reaction in genetically prone patients. Over time, the surgical wound healing is complicated with a keloid reaction, leading to formation of keloidal tissue along the lines of the sternotomy and other sites injured during the surgery. These keloids are often seen in elderly patients, as more cardiac surgeries are performed in later life.
Although there are several publications on this topic, to the author’s knowledge, to date no report of true incidence of this complication after thoracic surgery has been published. Such a report would highlight the prevalence of keloid disorder and provide a view into the epidemiology of the keloid disorder.
Searching PubMed for the keywords “Sternotomy Keloids” or “Thoracotomy Keloids” only resulted in small case series and anecdotal treatment results. Elliot’s (1985) study included a series of 100 consecutive sternotomy patients [8], but excluded patients who were African, Indian, or those with dark skin in order to assess the incidence of keloids in fair-skinned northern Europeans. This report concluded that among the 73 evaluable patients, no cases of true keloids were found. A similar study among Africans, African-Americans, or Asians is needed to provide insight into the frequency of keloid disorder or the development of post sternotomy keloids in each ethnic population.
Post sternotomy keloids not only form along the incision line (Figure 61), they can also form near the suture lines (Figure 62, 63), drain exit sites, and any other sites that sustain injury during thoracic surgery. Treatment of these keloids, especially when they present at advanced stages, is rather difficult. The author recommends early and aggressive intervention with ILT and ILC at the earliest signs of keloidal transformation of the sternotomy wounds.
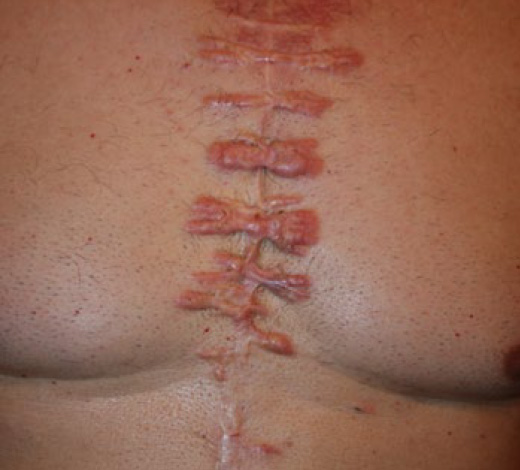
Figure 62. A 75-year-old Caucasian male presented 15 years after a sternotomy with numerous painful and pruritic patches of keloid, mostly along the suture lines. Note the near absence of keloid formation along the midline incision. Most keloids are formed along the horizontal suture lines.
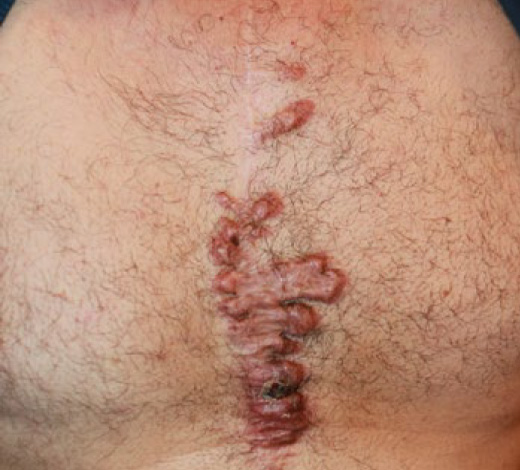
Figure 63. A 64-year-old Caucasian male who presented 14 years after a sternotomy with several pruritic and painful keloid patches mostly located along the suture lines, complicated with frequent infections and purulent discharge.
NEGATIVE IMPACT OF SURGERY
Treating chest keloid lesions, no matter how small the lesions may be, can be quite challenging. Although the option of surgery followed by radiation therapy might result in reasonable outcomes for some patients, the risk of recurrence and exposure to radiation in a young person cannot be ignored. As exemplified in the case studies presented earlier in this Guidance, reasonable outcomes can be achieved with non-surgical treatments. It is also reasonable to conclude that early implementation of nonsurgical treatments on early-stage and small chest keloid would result in better long-term outcomes.
Keloid removal surgery is a significant risk factor for the development of large tumoral or superficially spreading chest keloids. The injury from surgery to remove a small chest keloid can trigger formation of large tumoral keloids. Patients depicted in Figures 64-87 are examples of poor outcomes after surgery on anterior chest keloids.
Case Study 7
A 31-year old Hispanic female presented in October 2014 with a symptomatic anterior chest keloid that measured approximately 2×4 cm in diameter (Figure 65). The keloid’s margins were surrounded with inflammatory reaction in the adjacent skin. The keloid had been present for six years and was only treated with ILT, but with no appreciable response. Two small keloidal papules were also present in the periphery of the main keloid.
The patient was advised, and consented to treatment with a combination of ILT and cryotherapy. After three cycles of combined modality treatment, an overall reduction in the mass of the main keloid lesion was achieved along with noticeable improvement in the inflammatory reaction previously present in the lateral margins of this keloid. The most encouraging result was seen in the two small keloid papules, one below the main keloid and one above it. These papules had almost completely flattened and were no longer present after treatment (Figure 66).
At this junction (July 2015), the patient was unhappy with the partial results that we had achieved. ILC was offered to address the residual keloid tissue, however, the patient chose to switch her treatment course and instead decided to undergo surgery followed by radiation therapy.
In October 2017, the patient returned for re-evaluation and treatment of now recurrecnt post-operative keloids (Figure 67). During this visit, it was noted that the keloid papule previously in the inferior edge of the main keloid remained in remission. The papule located in the upper margin of the main keloid was removed surgically. Now after three years of follow up, the smaller lesion remained in remission after ILT and cryotherrapy.
This case exemplifies the difficulties encountered in treating anterior chest keloids, Post-operative recurrence remains a real dilemma for great majority of patients. Furthermore, with three years of follow up, this case is a good example of the efficacy of non-surgical treatment in eradicating keloid papules using ILT and cryotherapy.
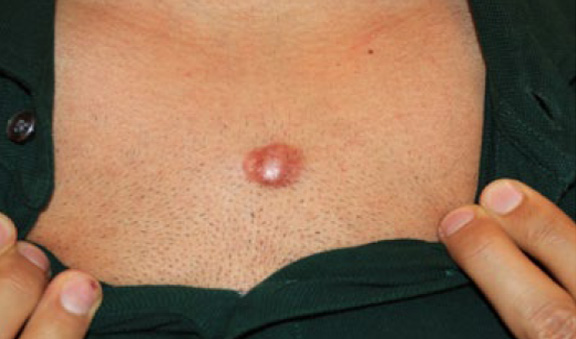
Figure 64. Keloid nodule with intense inflammatory borders in a 31-year-old Caucasian male. This keloid first started as a papule two years prior to this presentation. Keloid removal surgery was performed one year prior to this presentation. Unfortunately, there was a rapid recurrence after surgery.
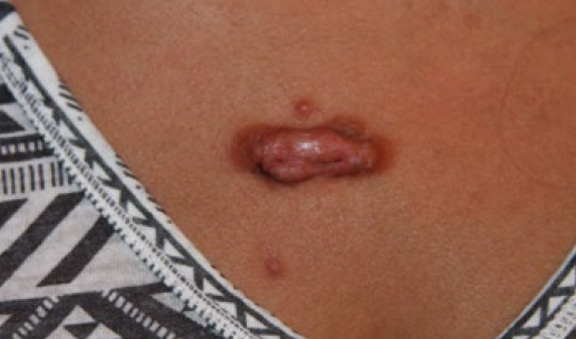
Figure 65. A 31-year-old Hispanic female (October 2014) with a tumoral anterior chest keloid. Note the presence of inflammatory reaction around the margin of the main keloid and multifocal disease with two papular keloids in the immediate vicinity of the main lesion.
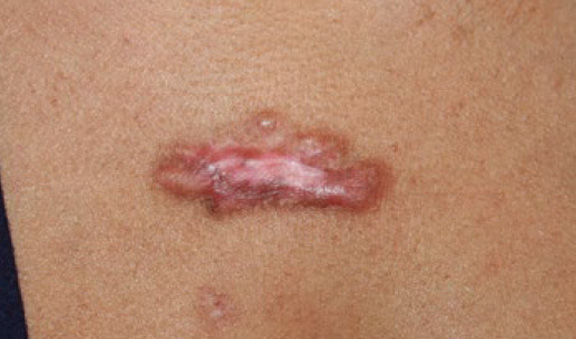
Figure 66. The same patient after recovery from three cycles of ILT and cryotherapy (July 2015). Note the thinning of the main keloid along with near-total flattening of the two keloid papules present at presentation.

Figure 67. The same patient after surgery and radiation therapy. Note the recurrent disease along the surgical incision line ( October 2017). Also, note that the small keloid papule previously present under the main keloid lesion, the one treated with ILT and cryotherapy, remained in remission with three years of follow up.
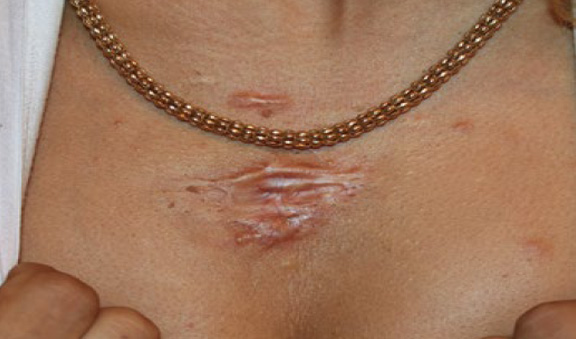
Figure 68. A 30-year-old African-American female with recurrent chest keloid after surgical removal of a small central chest keloid. Note the multifocality of the disease process.
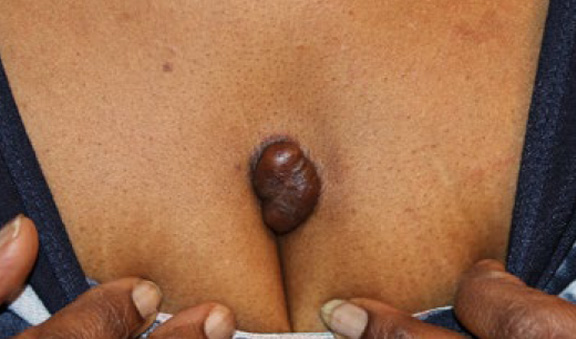
Figure 69. Post-operative recurrent tumoral chest keloid in a 60-year-old African-American female.
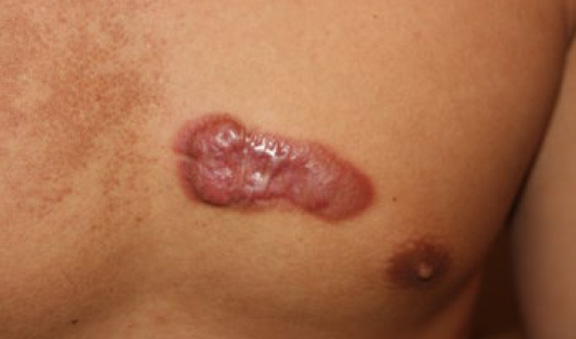
Figure 70. Post-operative recurrent tumoral chest keloid in a 36-year-old Caucasian male with an 18-year history of struggling with this instant chest keloid, with four failed attempts at surgery and several ILT injections.
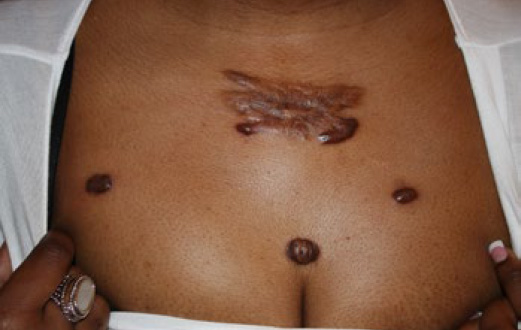
Figure 71. A 40-year-old African-American female with multifocal anterior chest keloids and recurrent disease after surgical removal of the keloid in her upper chest area.
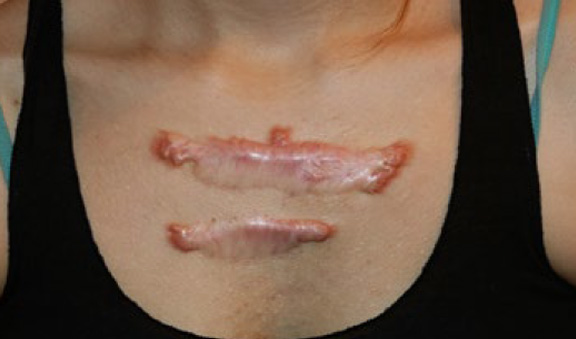
Figure 72. A 26-year-old Caucasian female with extremely painful central chest keloids that developed shortly after surgical removal of two small keloids.
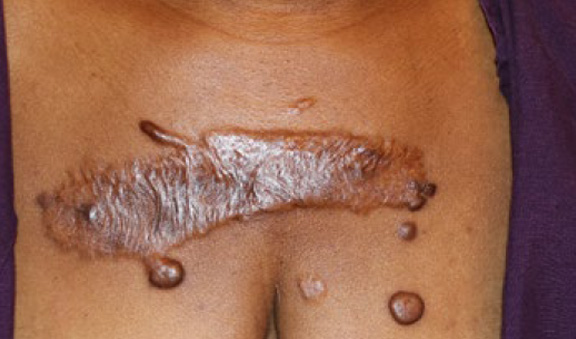
Figure 73. A 39-year-old African-American female with multifocal anterior chest keloids and recurrent disease after two surgical attempts and adjuvant radiation therapy.
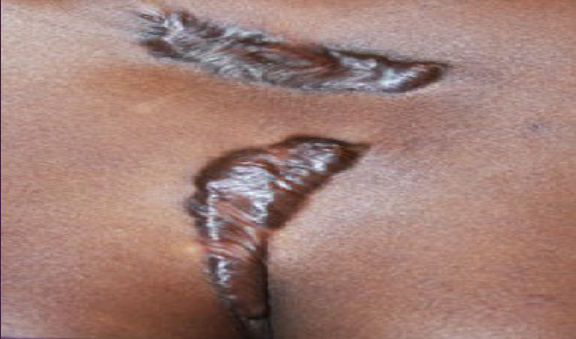
Figure 74. A 27-year-old African-American female with recurrent disease after surgical removal of two anterior chest keloids.
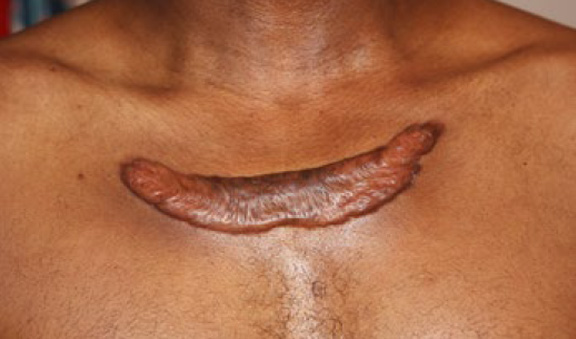
Figure 75. A 42-year-old African-American male with recurrent anterior chest keloid after surgical removal of an upper chest keloid.
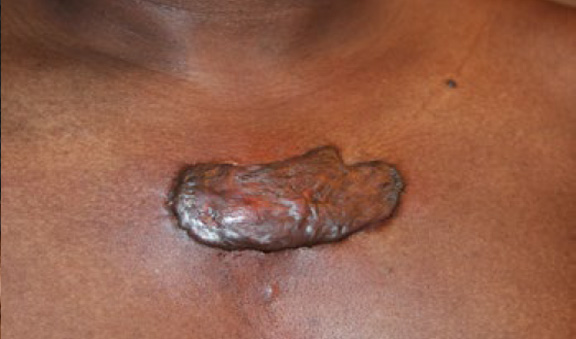
Figure 76. A 51-year-old African-American male with a ten-year history of keloid disorder. A smaller chest keloid was removed surgically six years earlier, which led to the formation of a large tumoral central chest keloid that has been constantly growing in size and complicated with frequent infections.
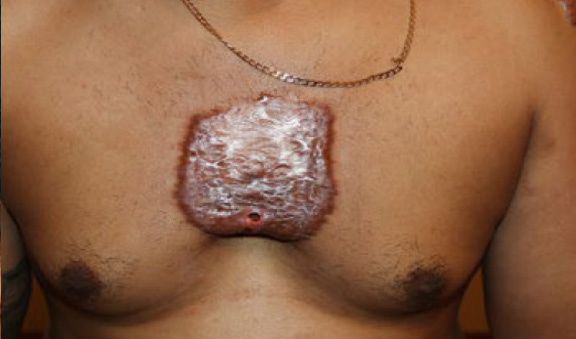
Figure 77. A 37-year-old African-American male with semimassive chest keloid that grew after the surgical removal of a smaller keloid. Note the presence of a non-healed draining fistula in the lower central part of this keloid. Frequent infections are among the most common commplications of massive chest keloids.
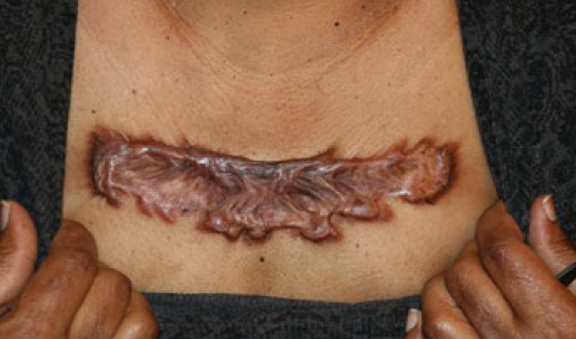
Figure 78. A 49-year-old African-American female with asemimassive chest keloid that grew after surgical removal of a smaller keloid.
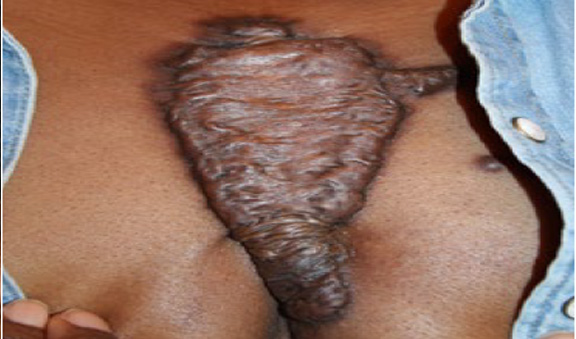
Figure 79. Massive central chest keloid in a 38-year-old African- American female who had previously undergone surgery to remove a small central chest keloid.
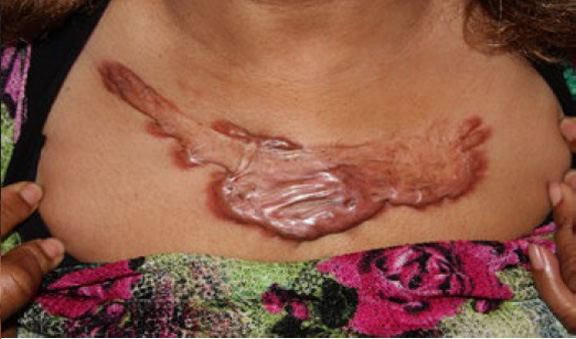
Figure 80. Massive central chest keloid in a 57-year-old African- American female who had previously undergone surgery to remove a much smaller central chest keloid.
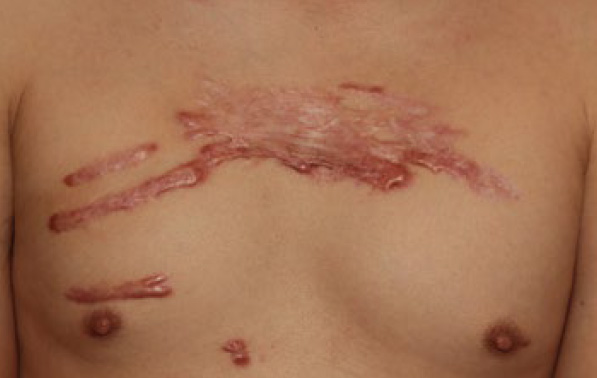
Figure 81. A 43-year-old Asian male with recurrent chest keloid despite prior treatment with surgery and radiation therapy. The multifocality of the disease strongly argues against the benefit from surgery, with or without radiation.
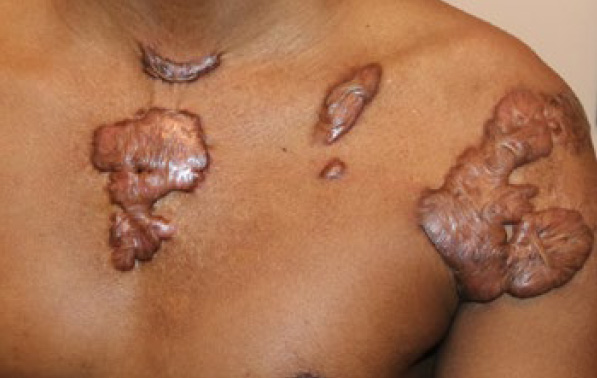
Figure 82. Multiple recurrent chest and shoulder keloids in a 34-year-old African-American male who had previously undergone surgery to remove keloids in the small central chest, lower neck, and left shoulder.
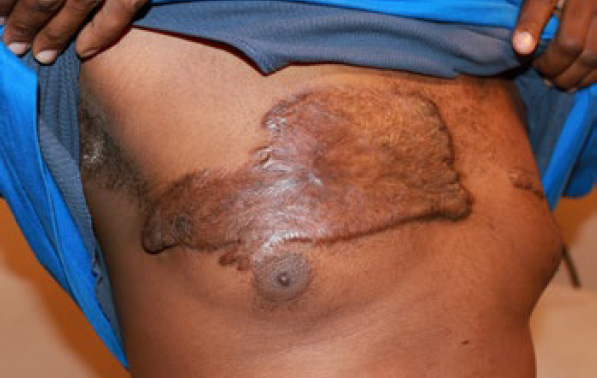
Figure 83. Superficially spreading anterior chest keloid in a 37-year-old African-American male who had previously undergone surgery to remove a small central chest keloid.
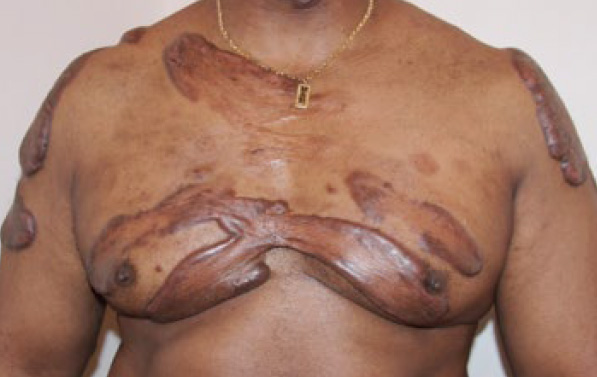
Figure 84. A 55-year-old male with extensive superficially spreading of chest wall and shoulder keloids, previously treated with multiple surgeries and adjuvant ILT injections.
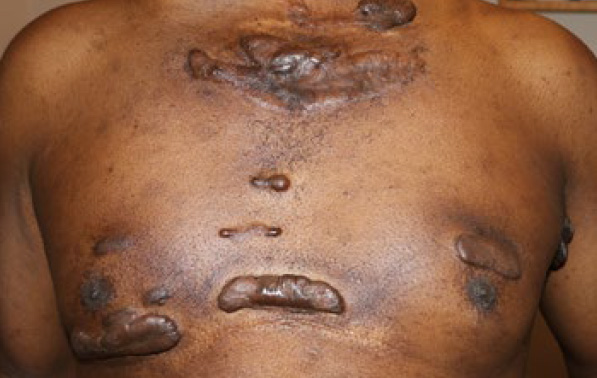
Figure 85. Post-operative recurrences in a 47-year-old male with extensive multifocal keloids since the age of 16. This patient had undergone surgery to remove several of his chest lesions followed with adjuvant ILT injections.
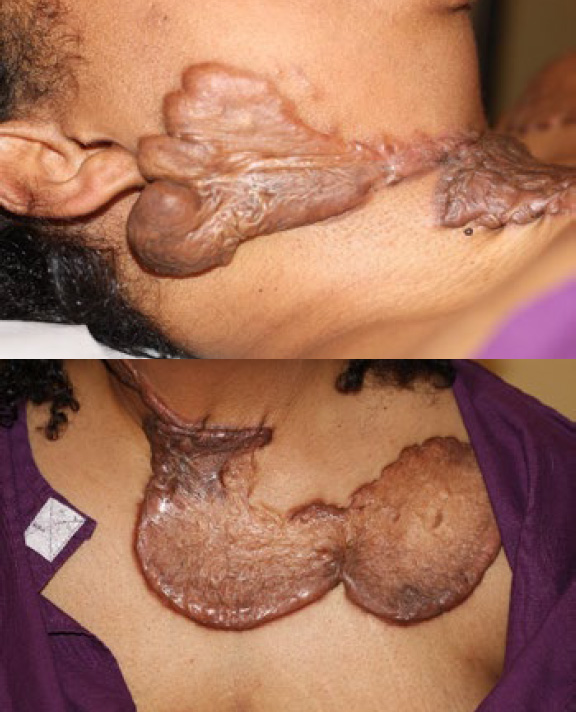
Figure 86. A 36-year-old old African-American female with massive neck and chest keloids. Note that her neck and chest keloids have merged, forming a very large, superficially-spreading keloid extending from her right ear to her neck, anterior chest, and left shoulder.
Case Study 8
A 36-year-old old African-American female presented with widespread keloid disorder involving numerous areas of her skin, including her neck and chest (Figure 86). Due to its importance, this case has also been discussed in the KRF Guidance for treatment of neck keloids.
This patient had previously undergone surgery and adjuvant radiation therapy to remove multiple keloids, including one from her neck and one from her chest. Unfortunately, the surgical intervention was followed by recurrence and development of new, yet much larger superficially spreading keloids, both in her neck and her anterior chest area. She also developed hypothyroidism after radiation therapy to her neck, for which she has been placed on life-long thyroid hormone supplement.
There are many lessons that we can learn from this one patient, and patients like her. Perhaps the most important is that surgery, with or without radiation therapy, is not a solution to this multifocal, genetic skin disorder. Contrary to the simplistic view that keloid lesions can be removed with surgery, this intervention quite often makes the condition significantly worse and causes permanent and lifelong complications.
The addition of radiation therapy to surgery not only proved ineffective, but also caused permanent hypothyroidism, another life-long iatrogenic complication imposed on this young woman. Radiation-induced hypothyroidism is an irreversible condition that requires lifelong thyroid hormone replacement [9]. Exposure to ionizing radiation is also a known risk factor for the development of thyroid cancer [10].
Treating patients like this young woman with such an extensive and life changing disease is extremely difficult. All such patients need a systemic form of treatment that unfortunately does not currently exist. Available treatments are inadequate to address the problems that anterior chest keloid patients suffer from.
BIOLOGY OF KELOID DISORDER
As evidenced in many cases presented in this Guidance, the pace of progression of anterior chest keloids is highly variable and for the most part unpredictable at onset. A study of patients who have had their disease for several years, however, indicates that there are well-defined subgroups of patients for whom the disease pace of progression is unique. The clinical data for all 484 patients seen by the author is being analyzed to determine the prevalence of each subtype of anterior chest keloids based on the progression pattern of the disorder. The following case studies clearly demonstrate the variability in the rate of growth and progression of anterior chest keloids among different patients.
Case Study 9
An 18-year-old Caucasian female presented in May 2014 with a two-year history of several papular keloids on her anterior chest (Figure 88) and both shoulders consistent with the diagnosis of DWACK syndrome. Acne was identified as the only triggering factor, which by age 18 had already improved.
Most of this patient’s keloids responded quite well to ILT. Figure 89 depicts her anterior chest keloids at her last follow up visit in February 2018. What is most interesting is that in this patient’s six-year history of keloid disorder, she had not developed any new keloid lesions. It appears as though the development of DWACK syndrome in this patient had a rapid onset, yet the disease process was brought to a halt with the control of the acne.
Case Study 10
A 20-year -old African-American female presented in April 2014 for treatment of ear keloids. During this visit, she was also found to have multiple keloids on her chest and shoulders. Figure 90 depicts presence of two tumoral anterior chest keloids at presentation. Later, this patient travelled to Europe to study. She was next seen in May 2017. Figure 91 depicts the appearance of the same anterior chest keloids three years later. The keloids have noticeably grown in size.
Case Study 11
A 23-year-old Indian male presented in August 2016 with several anterior chest (Figure 92) and bilateral shoulder keloids consistent with the diagnosis of DWACK syndrome. Figure 93 depicts a close-up view of the chest lesions at presentation. The patient reported that his disease had started approximately four years earlier and that he had previously been treated with several ILT injections and laser treatment. The patient also reported a very positive response to the laser treatments: all of his chest and shoulder keloid lesions showed significant improvement and were thinner and flat.
In light of the patient’s positive response to his current treatment, the patient was advised to return to the same dermatologist and continue with the same treatment regimen.
In January 2019, the patient returned to the author for a reevaluation, stating that the treatment he was receiving had stopped working and that his keloids had worsened. Figure 94 depicts the expansion and merger of several of the lesions that previously had responded to laser treatment.
This case, along with all other cases presented here, exemplify several facts about keloid disorder. Keloid disorder is a chronic, multi-focal, and dynamic genetic disorder of the skin. None of our current treatments can, or will, impact the disorder’s genetics or biology. It is naïve to think that successful removal of a keloid lesion, however, equates with a cure of the disease. Even if a keloid lesion in a patient responds to a particular treatment, the patient remains at risk of not only recurrence at the site, but also for developing new keloid lesions elsewhere on their skin.
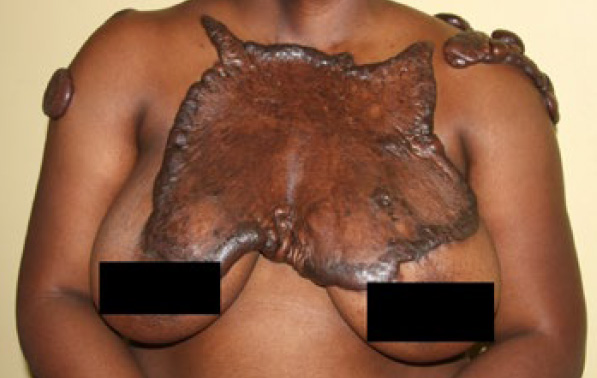
Figure 87. A 27-year-old African-American female with extensive superficially spreading chest wall keloid, previously treated with multiple surgeries and radiation therapy. Another example of how harmful and life-changing our treatments can be.
Case Study 12
A 25-year-old African-American male presented in April 2014 with multiple keloids involving his face, neck chest, and shoulders. His first keloids had developed at the age of six. Over the years, he had undergone several surgeries to remove keloids from his chest, face, and neck area, with some surgeries followed by radiation therapy. Figure 95 depicts the status of this patient’s anterior chest keloids at presentation. In contrast to several other cases presented bove, this patient clinically appeared to have been suffering from a much more aggressive form of keloid disorder. It is patients like this young man who are most in need of systemic treatments that we currently lack.
Figure 96 depicts the appearance of this patient’s chest keloids in August 2017. In a span of three years, all his chest keloids had grown in size, and several had indeed grown and merged with each other.
Figure 97 is the most recent follow up image that the patient submitted in January 2019. Notice significant expansion of all keloid lesions.
Clinical observation of the last four patients presented in case studies 9-12 reveals that keloid disorder can have a highly variable rate of growth and pattern in response to treatment.
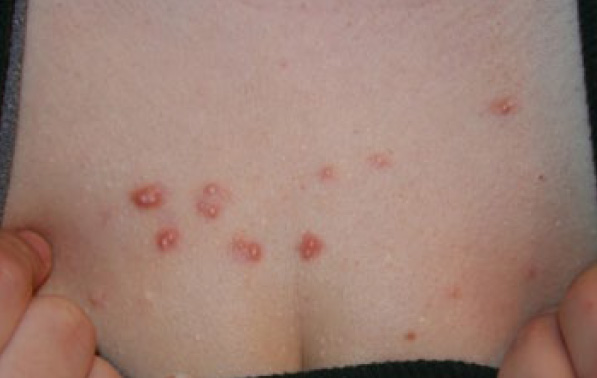
Figure 88. Multiple papular keloids of the anterior chest in an 18-year-old Caucasian female who has been struggling with keloid disorder for two years (May 2014).
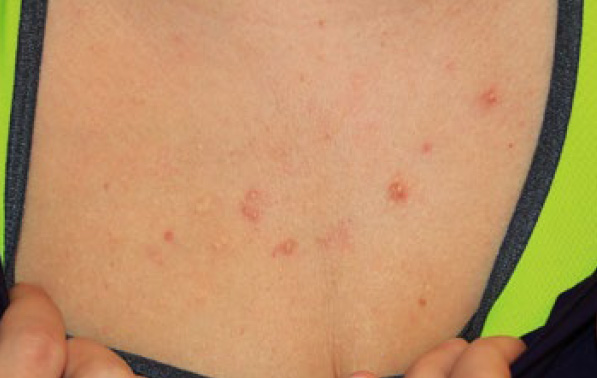
Figure 89. The same patient, four years later (February 2018). She has not developed any new lesions and treated lesions appear to be much improved.
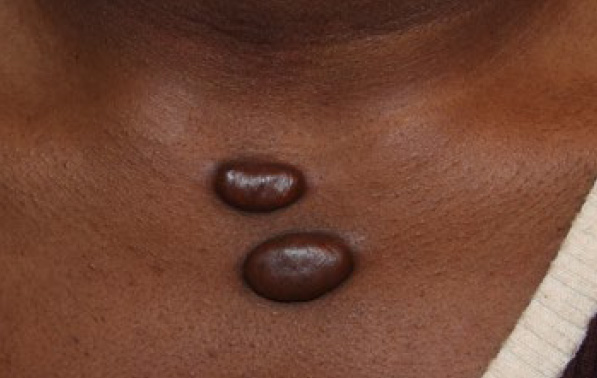
Figure 90. Two tumoral keloids of anterior chest wall in a 21-yearold African-American female (April 2014).
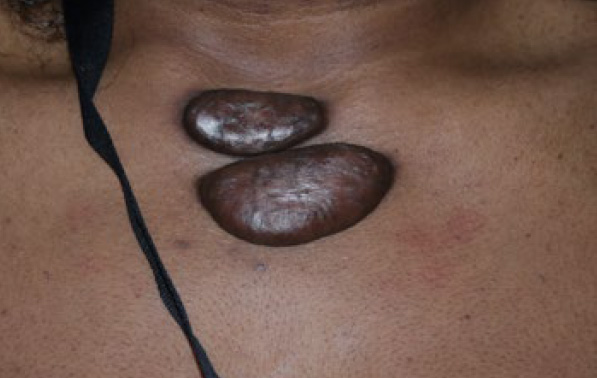
Figure 91. The same patient in April 2017, almost three years after her initial presentation. Note the significant growth and enlargement of the two anterior chest keloids present in 2014.
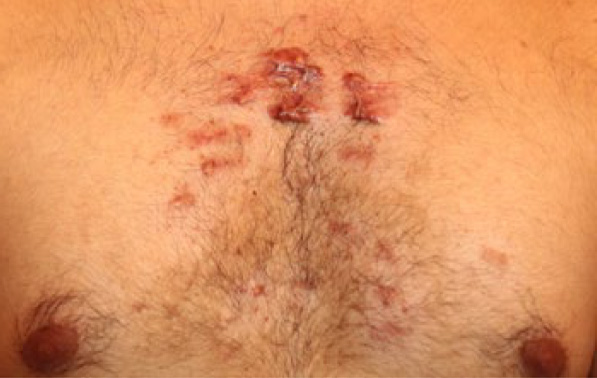
Figure 92. Anterior chest keloids in a 23-year-old Indian male (August 2016).
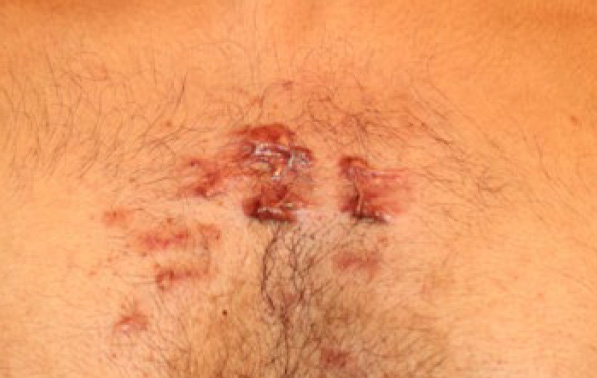
Figure 93. Close up view of the same lesions depicting response to the ongoing laser treatments. Several lesions appear to have diminished in size or totally flattened (August 2016).
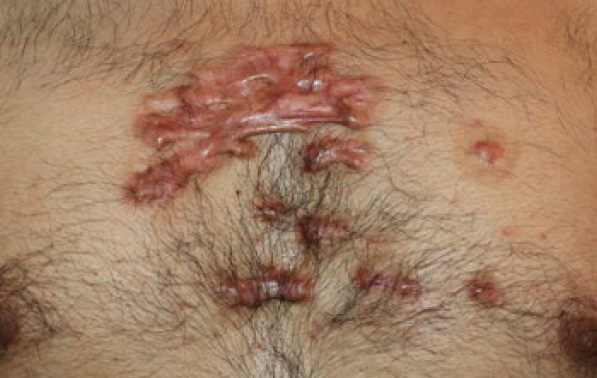
Figure 94. The same patient in January 2019. Note the progression of the disease and the formation of new lesions in a span of two and one-half years.
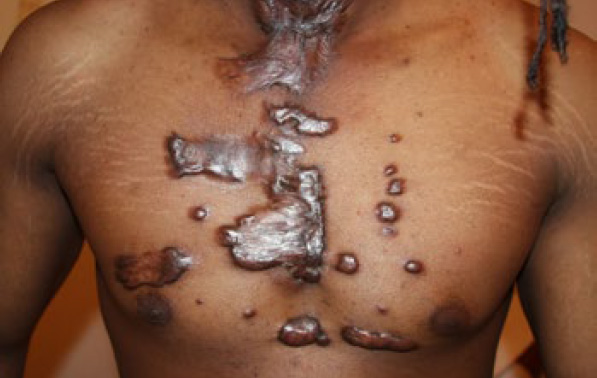
Figure 95. Numerous anterior chest keloids in a 25-year-old African- American male who had undergone several surgeries and radiation therapy to remove several of his chest wall keloids (April 2014).
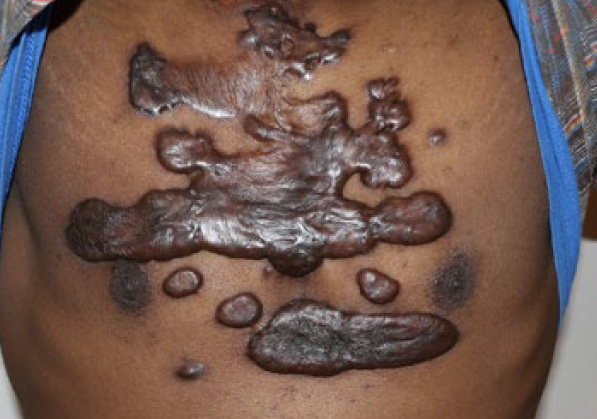
Figure 96. The same patient in August 2017. Note the significant progression of the disease in a span of three years.
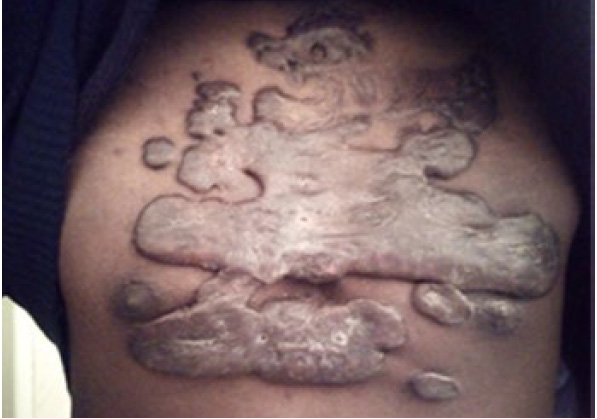
Figure 97. The same patient in January 2019. Note the significant progression of the disease in span of four and one-half years.
REFERENCES
- Tirgan, MH. Neck keloids: evaluation of risk factors and recommendation for keloid staging system, F1000 Research, June 28, 2016.
- Tirgan, MH. Massive ear keloids: Natural history, evaluation of risk factors and recommendation for preventive measures – A retrospective case series, F1000 Research, October 13, 2016.
- Miyahara H, Sato T, Yoshino K. Radiation-induced cancers of the head and neck region. Acta Otolaryngol Suppl. 1998;533:60-4.
- Ron E. Cancer risks from medical radiation. Health Phys. 2003 Jul;85(1):47-59.
- Tirgan, MH. Laser Treatment of Keloid Lesions, Efficacy and Side Effects, Results of an on-line survey. Abstract – 2nd International Keloid Symposium, Rome, Italy June 7-8 2018. Full manuscript in press.
- Ginarte M, Peteiro C, and Toribio J. 1999. “Keloid Formation Induced by Isotretinoin Therapy.” International Journal of Dermatology 38 (3): 228–29.
- Dogan G. 2006. “Possible Isotretinoin-Induced Keloids in a Patient with Behçet’s Disease.” Clinical and Experimental Dermatology 31 (4): 535–37.
- Elliot D, Cory-Pearce R, and Rees GM. 1985. “The Behaviour of Presternal Scars in a Fair-Skinned Population.” Annals of the Royal College of Surgeons of England 67 (4): 238–40.
- Boomsma MJ, et.al. A Prospective Cohort Study on Radiation-induced Hypothyroidism: Development of an NTCP Model. Int J Radiat Oncol Biol Phys. 2012 Nov 1;84(3):e351-6.
- Nachalon Y, Katz O, Alkan U, Shvero J, Popovtzer A. Radiation-Induced Thyroid Cancer: Gender-Related Disease Characteristics and Survival. Ann Otol Rhinol Laryngol. 2016
