IntralesIonal trIamcInolone acetonIde In the treatment of KeloId lesIons Can the treatment be harmful to some patients? Results of an online survey.
Michael H. Tirgan, MD
ABSTRACT
Background: Intralesional Triamcinolone Acetonide (ILTA) is the most commonly used method of treating keloidal lesions. Although there are several reports about the efficacy of ILTA, there is a paucity of literature as to how keloid patients perceive the effectiveness of this treatment modality.
Objective: To assess patients’ perception about the efficacy of ILTA in treatment of keloidal lesions.
Material and methods: Underlying study was approved by the Institutional Review Board (IRB). The online survey was launched in November 2011. Participants were asked to report their perception of the efficacy of ILTA for treatment of their keloidal lesions. Descriptive statistics are provided.
Results: As of May 29, 2017, a total of 1477 consecutive individuals participated in the survey. 847 participants indicated that they had previously received at least one ILTA, among whom 808 patients provided an assessment of the efficacy of this intervention. Nine patients (1.1%) reported that ILTA cured their keloids. 263 patients (32.6%) reported having benefited from the treatment. 396 patients (49%) reported no improvements, but most interestingly, 140 patients (17.3%) reported that ILTA caused worsening of their keloids.
Conclusions and Relevance: With several limitations, this study represents the first step in developing a patient reported measure of treatment success and benefit drawn from ILTA. The most important finding of this study is that 17.3% of patients reported that worsening of their keloids with this treatment. Worsening of keloids after steroid injections has never been reported.
INTRODUCTION
Although clinical studies have assessed many treatments for keloid disorder, including ILTA, the heterogeneity of these studies makes comparison of the effectiveness of this treatment – alone or combined with other interventions – very difficult.
Applied intralesionally, triamcinolone acetonide (TA) is one of the most commonly used drugs for treatment for keloidal lesions1,2. Sexton reported the efficacy of intradermal injection of this fluorinated prednisolone derivative in 19603. Hussain in his recent publication states that “steroid injection is still one of the most effective methods of treating keloid scars”4. Atiyeh also affirms that ILTA plays a significant role in the regression of hypertrophic scars and keloids5.
Although used very commonly, TA may not be as effective or as easy to tolerate. Muneuchi et. al studied the long-term outcome of ILTA into keloid lesions in Asian patients6. Between 1985 and 2003, they treated 94 patients by injecting 1 to 10 mg of TA, depending on the size of the lesion, at four week intervals. Thirty-one patients (33%) gave up treatment within 10 injections because of pain and lack of benefit. Improvement in subjective symptoms was seen in 52 patients (55%). In objective symptoms, fair or better results were seen in 40 patients (43%), and good or better results in 25 patients (27%). The treatment method required 20-30 injections over three to five years.
Wong et al in their 2016 meta-analysis of the all published literature on usage of ILTA in treatment of keloids identified a total of 113 studies. They were, however, able to use only eight studies with a total of 359 patients and chose to exclude the rest for lack of reporting outcomes, or reporting data that was previously reported elsewhere7. The dose/concentration of TA used across these eight studies varied from 10mg/ml to 40 mg/ml. The authors concluded that “given that triamcinolone treatment will lead to substantial side effects, selection between this drug and other treatment options should be evidence based and the discernible benefits shall be clarified.”
An IRB approved online keloid survey was launched by the author in November 2011 to inquire about various aspects of keloid disorder, including the efficacy and potential side effects of various treatment modalities, including ILTA. The author hereby reports on patients’ perceptions among 808 survey participants who had been treated with ILTA. To the author’s knowledge, this is the largest study ever conducted about the efficacy, or lack thereof, of ILTA in treatment of keloid patients.
INSTITUTION
Keloid Research Foundation
23 West 73rd Street,
Suite GD, New York,
NY 10023
(646) 320 7256
CONFLICT OF INTEREST
None
FUNDING
None
IRB ASSESSMENT
The online survey was approved by IRB (KD)
KEYWORDS
Keloid, intralesional steroids, triamcinolone
MATERIAL AND METHODS
A comprehensive questionnaire was developed to survey a large cohort of consecutive unselected patients with keloid disorder. The study was initially approved in November of 2011 by the Institutional Review Board (IRB) of St. Luke’s-Roosevelt Hospital in New York. The study was subsequently transferred to Western IRB. Potential participants in the study were asked to access the study questionnaire by visiting the study website, www.KeloidSurvey.com. After downloading and reviewing the study consent form, adult participants were asked to acknowledge the informed consent electronically. Parents could consent and complete the survey on behalf of their underage children. Th e survey posed numerous questions to the participants, assessing a patient’s age, ethnic background, family history, extent and distribution pattern of the keloid lesions, prior treatments and response rates. Participants’ access to survey tool was limited to one access per computer IP address. Participants were allowed to skip questions, either because the question did not apply to them, or they simply did not wish to provide an answer to a particular question. In this manuscript, the author reports the perception of the survey participants of the benefit they gained from steroid injections. The study dataset was accessed on April 29, 2017. Descriptive statistics are presented.
Results
The study was opened for accrual on November 14, 2011. As of May 29, 2017, a total of 1477 individuals participated in this survey. 847 participants indicated that they had previously received at least one ILTA. 835 participants disclosed their gender among whom 265 participants (31.7%) were male and 570 (68.3%) were female. Basic demographic characteristics of the participants are as follows
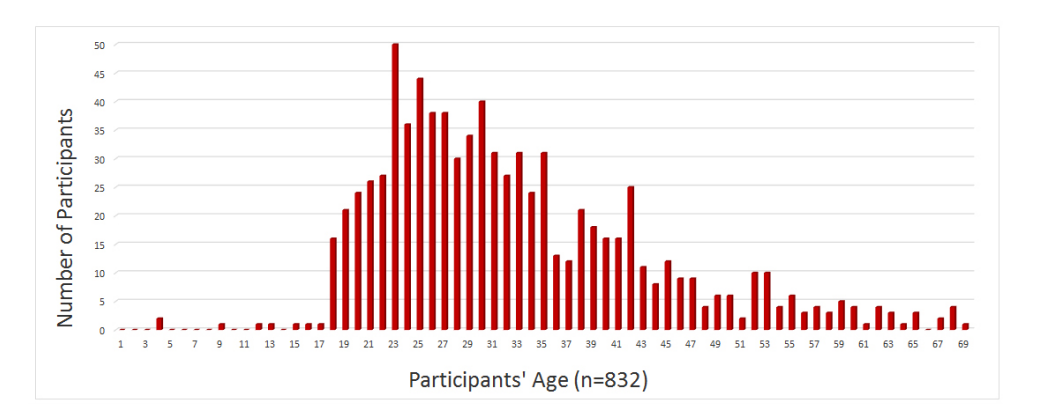
Age
Participants were asked to provide their age. In addition, they were also asked to recall the age when they developed their very first keloid. Figure 1 depicts participants’ age at the time they took the survey as well as the age of onset of their keloid disorder. Peak age of onset of keloid disorder was 16
Country of Birth
A total of 837 participants provided information about their country of birth. Majority of participants (58.5%)

were born in United States, 6.6% were born in India,
2.6% were born in United Kingdom, 2.5% were born
in Malaysia and 2.5% in China. Figure 2 depicts the
country of birth of all study participants.
Although this distribution pattern correctly represents
the country of birth of those who participated in this study,
it is by no means a true reflection of the epidemiology of
keloid disorder. Information about the country of birth
of participants is most likely a reflection of the level of
healthcare services that might have been available to the
participants. However, this information does not take
into consideration the migration patterns, neither does it
reflect of the country of residence of the participants.

Ethnicity
Participants were asked to provide their ethnic background. A total of 843 participants provided this information. Figure 3 shows percentages for different
ethnic characteristics of the respondents. Although this information is a correct representation of those who participated in this study, it is not a true reflection of the ethnic epidemiology of keloid disorder.

Pattern of Distribution of
Keloid Lesions
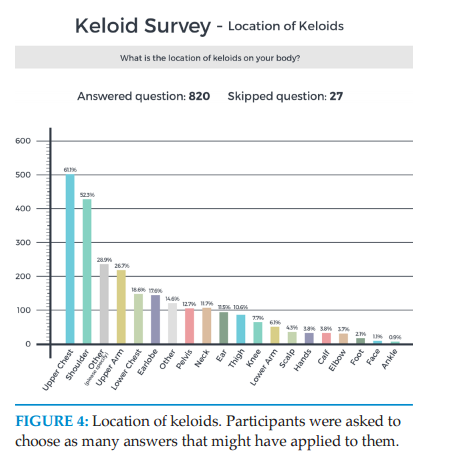
Participants were asked to provide detailed information
about distribution of the keloid lesions throughout their
skin. Upper chest, shoulders and upper arms were the
most frequently involved area of the skin among the
study participants. Figure 4 depicts the distribution
patterns of keloid lesions among the study participants.
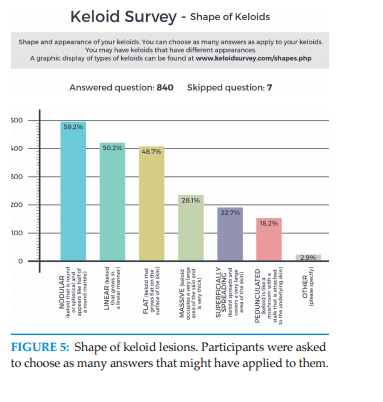
Appearance and Shape of Keloid Lesions
Participants were asked to describe the shape and the
appearance of their keloid lesions. To facilitate this, a
reference guide was provided online. As seen in Figure 5,
nodular, linear and flat keloids were the most common
forms of keloidal lesions among the participants. 28%
of patients considered their keloids to be massive, with
keloid lesions occupying large areas of their skin.
Triggering factors
Participants were asked to provide information about the factors that triggered formation of their keloids. 794 participants provided answers to this inquiry.
Figure 6 shows the frequency of various triggering factors within this population. Acne was by far the most common
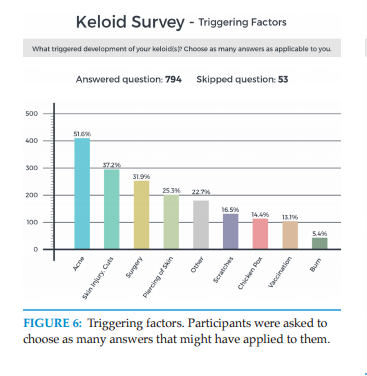
triggering factor, followed by skin injury and surgery. 14.4% of participants listed chicken pox and 13.1% listed vaccinations as the triggering factor for development of their keloids.
Intralesional Triamcinolone Acetonide Injections
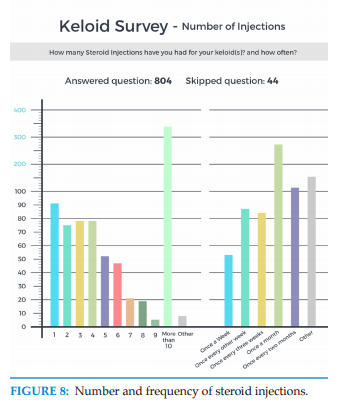
Participants were asked to recall the total number of steroid injections. It was assumed that participants would not be familiar with the term “ILTA”, and therefore the term “steroid injection” was used in the survey tool. Furthermore, the author assumed that all patients were treated with TA or an equipotent steroid. Participants were also asked to recall the frequency of the injections they had received. As depicted in Figure 8, the survey relied on patients’ understanding and verification of the treatment they had received. Survey did not ask patients about the type of the steroid, or the dosage of the drug that was injected. It is interesting to note that a large number of patients reported receiving more than 10 injections, and often on a monthly schedule.
Efficacy of ILTA
Efficacy of ILTA was assessed by asking participants to provide only one answer to the following three multiplechoice questions.
Question #1
What was the outcome of steroid injections on your keloid(s)?
1. After steroid injection(s) my keloid(s) resolved and
disappeared and never came back.
2. After steroid injection(s) my keloid(s) improved yet
they grew back but remained smaller than before
injections.
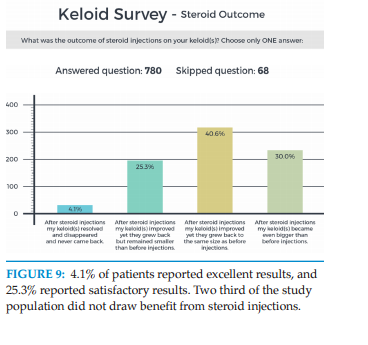

3. After steroid injection(s) my keloid(s) improved yet
they grew back to the same size as before injections.
4. After steroid injection(s) my keloid(s) became even
bigger than before injections.
Question #2
Your assessment of benefit you had from steroid
Injections.
1. Steroid injections cured my keloid(s).
2. Steroid injections were definitely helpful and made
my keloid(s) better.
3. Steroid injections did not improve my keloid(s).
4. Steroid injections made my keloid(s) worse.
Participants’ answers to question #2 discovered a totally
new finding. 17.3% of participants reported that their
keloids worsened after steroid injections and they
contributed this worsening to the steroid injections.
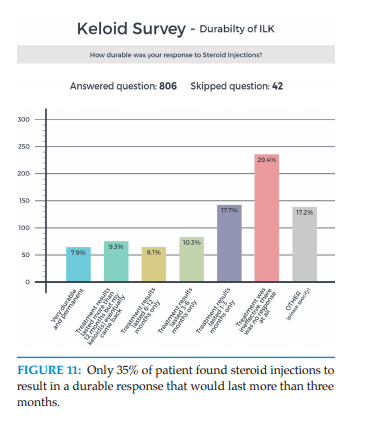
Question #3
How durable was your response to steroid Injections?
1. Very durable and permanent.
2. Treatment results lasted more than 12 months but
my keloid(s) eventually came back.
3. Treatment results lasted 6-12 months only.
4. Treatment results lasted 3-6 months only
5. Treatment results lasted 1-3 months only.
6. Treatment was ineffective; there was no response at all.
DISCUSSION
This study has a number of strengths, most importantly its sample size of 847 participants, making this the largest study ever conducted about the efficacy, or lack thereof, of ILTA in treatment of keloid patients. The large sample size also allowed for an analysis of associations between respondent characteristics and their survey responses. However, there are also several limitations. First, this was not a random survey of the general population of patients with keloid disorder, but was biased towards those searching the web for information related their illness, or possible treatment thereof. Second, there could be overrepresentation of younger and more computer literate age groups and under-representation of those who do not speak English, as well as those living in communities without access to internet. Third, the survey may have not been as inclusive of all potential or controversial characteristics of the variables that were analyzed. Fourth, the survey tool has never been validated before.
Nevertheless, this self-selected group of respondents who answered the survey questions, provide us with a glimpse into the real world experience of those suffering from keloids, and their perception as to the efficacy of ILTA, or lack thereof.
ILTA is often used as the primary mode of treatment for patients with early-stage keloids or those with numerous small keloidal lesions. It is also used as an adjunct following surgical excision of keloidal lesions. Injections are felt to cause low morbidity, be cost effective and easy to administer and provide reliable and durable results8. Insalco et. al reported that “steroids have proven to be effective as monotherapy on multiple occasions” 9, quoting Darzi et al. who had performed a review of multiple treatment modalities for keloids, including ILTA as a single-agent modality10. Darzi et al. reported that ILTA produced symptomatic relief in 72%, and complete flattening in 64% of the lesions. However, not all keloid lesions will respond to ILTA. Some authors have even questioned patients’ ability to comply with the treatment regimen, given the pain and the long treatment course that is often required5. There is also no data as to how keloid patients perceive the efficacy of this intervention. In contrast to the published literature that generally reports a positive assessment by the investigators9,10, the data from this very large study indicates that ILTA is not perceived by the patients to be as effective as it is reported in the medical literature. Even when effective, the efficacy of this treatment modality was not durable and faded over time, with only 35% patients seeing results that lasted over three months, and nearly 65% of patient drawing either no benefit, or very minimal benefit that lasted only one to three months. Most importantly, and for the first time, this study revealed that steroid injections can actually be harmful to some patients and cause worsening of keloids. The fact that only some patients benefit from ILTA argues in favor of existence of a subset of keloid lesions that are steroidsensitive. However, there must also exist some patients, or some keloidal lesions that are steroid-resistant. Injecting inside the keloid tissue does result in dissection of the tissue and induction of a new injury. This iatrogenic tissue injury within a keloidal tissue that is inherently resistant to TA may indeed be the triggering factor leading to the worsening and aggravation of the keloidal lesion. The mechanisms involved in sensitivity or resistance of keloids to intralesional steroids are not known. Identifying such mechanisms and understanding the biological pathways that lead to sensitivity, or resistance to steroids may open doors to discovery of better treatments for keloid disorder.
It is important that outcomes used in clinical research are relevant, not only to the clinicians, but also to the patients. Partial
regression of a keloid lesion is a measurable outcome. Yet, this particular endpoint may not be so important when it is interpreted as a poor cosmetic result by a patient. In a systematic review of outcome measures used in assessing efficacy of treatment intralesional treatment for hypertrophic scars and keloids, Perdanasari et. all concluded that “the literature itself is confusing because there is no standardized method of reporting results” (11).
CONCLUSION
Although this study has certain limitations, its large sample size lends credibility to its findings. ILTA was found to be effective only in one third of cases, and led to worsening of keloids in 17.3% of patients. Patients’ perceptions of the beneficial or detrimental effects of treatment are two crucial factors that determine patients’ compliance and adherence to treatment. False expectations are likely to result in patients’ disappointment and poor treatment outcomes.
Patient-reported outcomes have not been commonly used in keloid treatment trials and validated tools are lacking. The author recommends inclusion of patients’ views, and patients’ reported outcomes, in all clinical trials evaluating interventions for treatment of keloid disorder.
There is a paucity of data about every aspect of keloid disorder. Most importantly, practitioners lack datadriven and evidence-based treatment guidelines. The author encourages the readers to provide the link to the website of this ongoing study - www.KeloidSurvey.com - to all their keloid patients.
REFERENCES
1. Wolfram D, Tzankov A, Pulzl P, Riza-Katzer H. Hypertrophic scars and keloids: review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171-181.
2. Hochman B, Locali RF, Matsuoka PK, Ferreira LM. Intralesional triamcinolone acetonide for keloid treatment: a systematic review. Aesth Plast Surg. 2008;32:705-709.
3. Sexton GB. Local injection of triamcinolone acetonide in the management of certain skin conditions, preliminary report. Can Med Assoc J. 1960;83(26):1379-1381.
4. Hussain MA, Kuruppu L, Sarhadi N. Step-by-step guide of a technique of using dental cartridges for intralesional steroid injection to keloids. Eur J Plast Surg. 2011; 34:307-309.
5.Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31:468- 492.
6. Muneuchi G, Suzuki S, Onodera M, Ito O, Hata Y, Igawa HH. Long-term outcome of intralesional injection of triamcinolone acetonide for the treatment of keloid scars in Asian patients. Scand J Plast Reconstr Surg Hand Surg. 2006;40:111-6.
7. Wong TS, Li JZ, Chen S, Chan JY, Gao W. The efficacy of triamcinolone acetonide in keloid treatment: A systematic review and meta-analysis. Front Med (Lausanne). 2016 Dec 27;3:71.
8. Park TH, Seo SW, Kim JK, et al. Clinical characteristics of facial keloids treated with surgical excision followed by intra- and postoperative intralesional steroid injections. Aesthetic Plast Surg. 2012 Feb;36(1):169-73.
9. Insalaco L, Saxon S, Spiegel JH. What is the role of intralesional corticosteroid injections for keloids before considering surgery? Laryngoscope. 2016 Mar;126(3):549-50
10. Darzi MA, Chowdri NA, Kaul SK, Khan M. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg 1992;45:374–379.
11. Perdanasari A, Torresetti M, Grassetti L, Nicoli F, Zhang YX, Dashti T, Di Benedetto G, Lazzeri D. Intralesional injection treatment of hypertrophic scars and keloids: a systematic review regarding outcomes. Burns Trauma. 2015 Aug 26;3:14.
